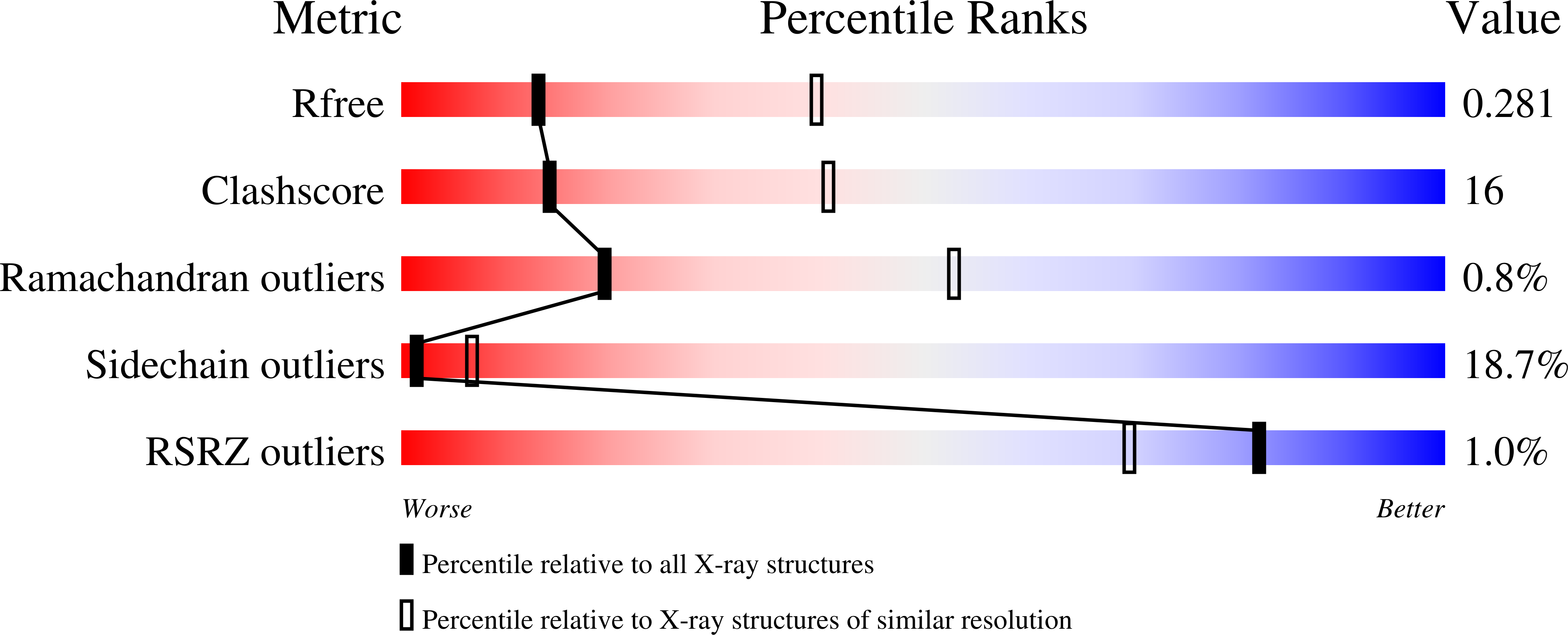
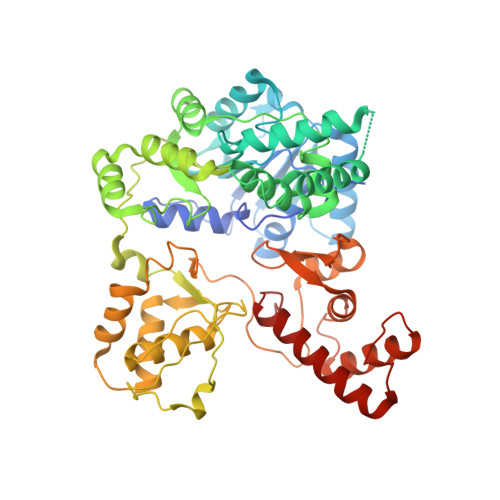
Structural Insights into Substrate Recognition and Catalysis in Outer Membrane Protein B (OmpB) by Protein-lysine Methyltransferases from Rickettsia.
Abeykoon, A.H., Noinaj, N., Choi, B.E., Wise, L., He, Y., Chao, C.C., Wang, G., Gucek, M., Ching, W.M., Chock, P.B., Buchanan, S.K., Yang, D.C.(2016) J Biol Chem 291: 19962-19974
- PubMed: 27474738
- DOI: https://doi.org/10.1074/jbc.M116.723460
- Primary Citation of Related Structures:
5DNK, 5DO0, 5DOO, 5DPD, 5DPL - PubMed Abstract:
Rickettsia belong to a family of Gram-negative obligate intracellular infectious bacteria that are the causative agents of typhus and spotted fever. Outer membrane protein B (OmpB) occurs in all rickettsial species, serves as a protective envelope, mediates host cell adhesion and invasion, and is a major immunodominant antigen. OmpBs from virulent strains contain multiple trimethylated lysine residues, whereas the avirulent strain contains mainly monomethyllysine. Two protein-lysine methyltransferases (PKMTs) that catalyze methylation of recombinant OmpB at multiple sites with varying sequences have been identified and overexpressed. PKMT1 catalyzes predominantly monomethylation, whereas PKMT2 catalyzes mainly trimethylation. Rickettsial PKMT1 and PKMT2 are unusual in that their primary substrate appears to be limited to OmpB, and both are capable of methylating multiple lysyl residues with broad sequence specificity. Here we report the crystal structures of PKMT1 from Rickettsia prowazekii and PKMT2 from Rickettsia typhi, both the apo form and in complex with its cofactor S-adenosylmethionine or S-adenosylhomocysteine. The structure of PKMT1 in complex with S-adenosylhomocysteine is solved to a resolution of 1.9 Å. Both enzymes are dimeric with each monomer containing an S-adenosylmethionine binding domain with a core Rossmann fold, a dimerization domain, a middle domain, a C-terminal domain, and a centrally located open cavity. Based on the crystal structures, residues involved in catalysis, cofactor binding, and substrate interactions were examined using site-directed mutagenesis followed by steady state kinetic analysis to ascertain their catalytic functions in solution. Together, our data reveal new structural and mechanistic insights into how rickettsial methyltransferases catalyze OmpB methylation.
Organizational Affiliation:
From the Department of Chemistry, Georgetown University, Washington, D. C. 20057.