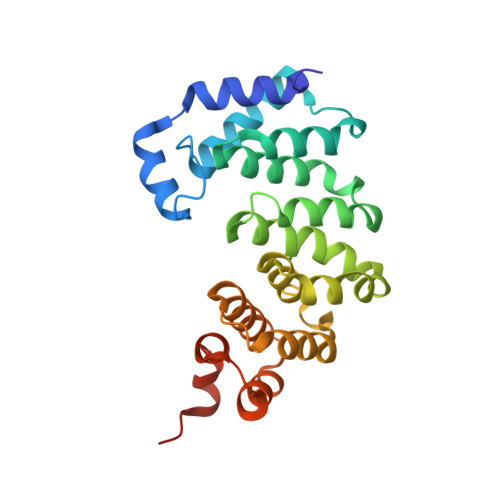
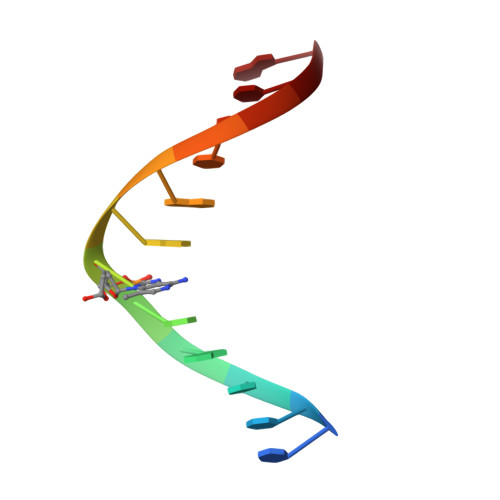
The DNA glycosylase AlkD uses a non-base-flipping mechanism to excise bulky lesions.
Mullins, E.A., Shi, R., Parsons, Z.D., Yuen, P.K., David, S.S., Igarashi, Y., Eichman, B.F.(2015) Nature 527: 254-258
- PubMed: 26524531
- DOI: https://doi.org/10.1038/nature15728
- Primary Citation of Related Structures:
5CL3, 5CL4, 5CL5, 5CL6, 5CL7, 5CL8, 5CL9, 5CLA, 5CLB, 5CLC, 5CLD, 5CLE - PubMed Abstract:
Threats to genomic integrity arising from DNA damage are mitigated by DNA glycosylases, which initiate the base excision repair pathway by locating and excising aberrant nucleobases. How these enzymes find small modifications within the genome is a current area of intensive research. A hallmark of these and other DNA repair enzymes is their use of base flipping to sequester modified nucleotides from the DNA helix and into an active site pocket. Consequently, base flipping is generally regarded as an essential aspect of lesion recognition and a necessary precursor to base excision. Here we present the first, to our knowledge, DNA glycosylase mechanism that does not require base flipping for either binding or catalysis. Using the DNA glycosylase AlkD from Bacillus cereus, we crystallographically monitored excision of an alkylpurine substrate as a function of time, and reconstructed the steps along the reaction coordinate through structures representing substrate, intermediate and product complexes. Instead of directly interacting with the damaged nucleobase, AlkD recognizes aberrant base pairs through interactions with the phosphoribose backbone, while the lesion remains stacked in the DNA duplex. Quantum mechanical calculations revealed that these contacts include catalytic charge-dipole and CH-π interactions that preferentially stabilize the transition state. We show in vitro and in vivo how this unique means of recognition and catalysis enables AlkD to repair large adducts formed by yatakemycin, a member of the duocarmycin family of antimicrobial natural products exploited in bacterial warfare and chemotherapeutic trials. Bulky adducts of this or any type are not excised by DNA glycosylases that use a traditional base-flipping mechanism. Hence, these findings represent a new model for DNA repair and provide insights into catalysis of base excision.
Organizational Affiliation:
Department of Biological Sciences and Center for Structural Biology, Vanderbilt University, Nashville, Tennessee 37232, USA.