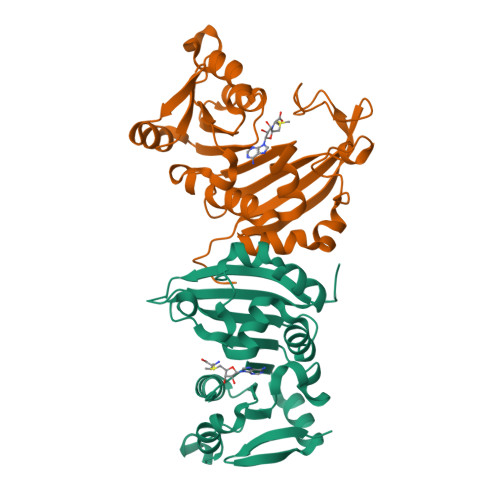
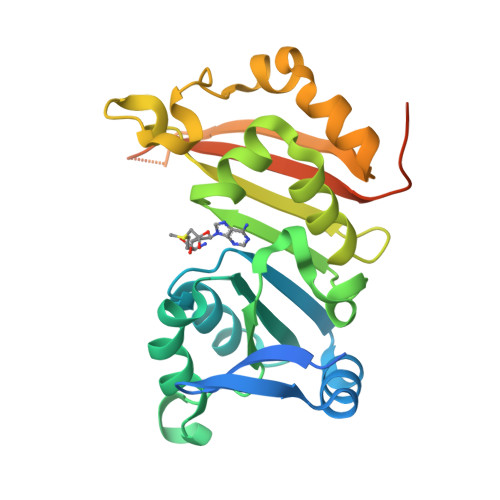
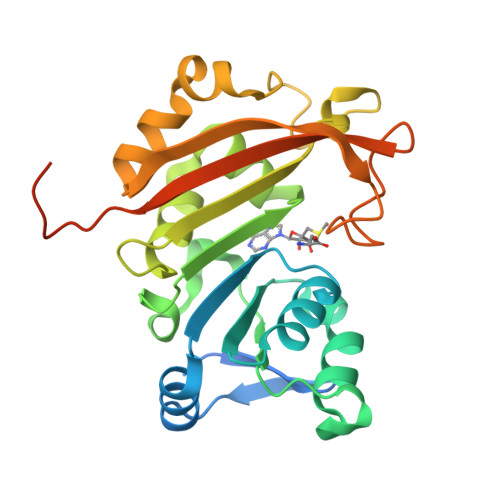
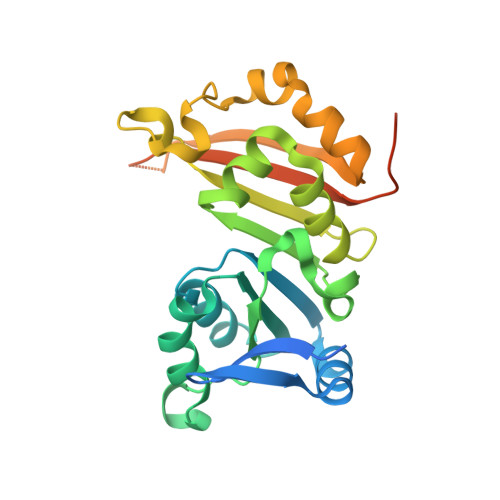
Functional dichotomy in the 16S rRNA (m1A1408) methyltransferase family and control of catalytic activity via a novel tryptophan mediated loop reorganization.
Witek, M.A., Conn, G.L.(2016) Nucleic Acids Res 44: 342-353
- PubMed: 26609134
- DOI: https://doi.org/10.1093/nar/gkv1306
- Primary Citation of Related Structures:
4X1O, 5BW4, 5BW5, 5D1H, 5D1N - PubMed Abstract:
Methylation of the bacterial small ribosomal subunit (16S) rRNA on the N1 position of A1408 confers exceptionally high-level resistance to a broad spectrum of aminoglycoside antibiotics. Here, we present a detailed structural and functional analysis of the Catenulisporales acidiphilia 16S rRNA (m(1)A1408) methyltransferase ('CacKam'). The apo CacKam structure closely resembles other m(1)A1408 methyltransferases within its conserved SAM-binding fold but the region linking core β strands 6 and 7 (the 'β6/7 linker') has a unique, extended structure that partially occludes the putative 16S rRNA binding surface, and sequesters the conserved and functionally critical W203 outside of the CacKam active site. Substitution of conserved residues in the SAM binding pocket reveals a functional dichotomy in the 16S rRNA (m(1)A1408) methyltransferase family, with two apparently distinct molecular mechanisms coupling cosubstrate/ substrate binding to catalytic activity. Our results additionally suggest that CacKam exploits the W203-mediated remodeling of the β6/7 linker as a novel mechanism to control 30S substrate recognition and enzymatic turnover.
Organizational Affiliation:
Department of Biochemistry, Emory University School of Medicine, Atlanta GA 30322, USA.