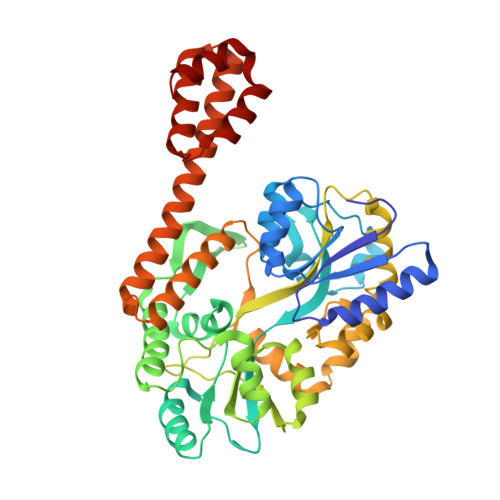
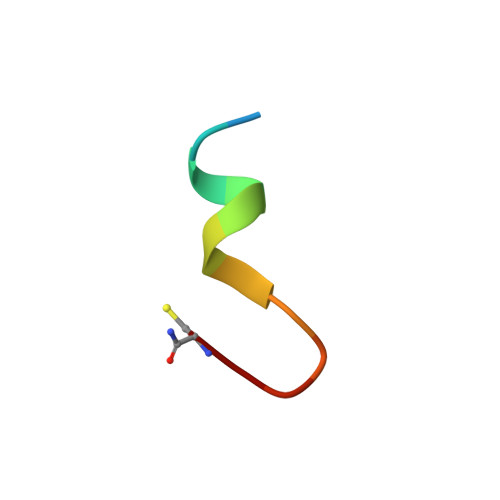
Rational design of crystal contact-free space in protein crystals for analyzing spatial distribution of motions within protein molecules.
Matsuoka, R., Shimada, A., Komuro, Y., Sugita, Y., Kohda, D.(2016) Protein Sci 25: 754-768
- PubMed: 26694222
- DOI: https://doi.org/10.1002/pro.2867
- Primary Citation of Related Structures:
5AZ6, 5AZ7, 5AZ8, 5AZ9, 5AZA - PubMed Abstract:
Contacts with neighboring molecules in protein crystals inevitably restrict the internal motions of intrinsically flexible proteins. The resultant clear electron densities permit model building, as crystallographic snapshot structures. Although these still images are informative, they could provide biased pictures of the protein motions. If the mobile parts are located at a site lacking direct contacts in rationally designed crystals, then the amplitude of the movements can be experimentally analyzed. We propose a fusion protein method, to create crystal contact-free space (CCFS) in protein crystals and to place the mobile parts in the CCFS. Conventional model building fails when large amplitude motions exist. In this study, the mobile parts appear as smeared electron densities in the CCFS, by suitable processing of the X-ray diffraction data. We applied the CCFS method to a highly mobile presequence peptide bound to the mitochondrial import receptor, Tom20, and a catalytically relevant flexible segment in the oligosaccharyltransferase, AglB. These two examples demonstrated the general applicability of the CCFS method to the analysis of the spatial distribution of motions within protein molecules.
Organizational Affiliation:
Division of Structural Biology, Medical Institute of Bioregulation, Kyushu University, Higashi-Ku, Fukuoka, 812-8582, Japan.