TNIK inhibition abrogates colorectal cancer stemness
Masuda, M., Uno, Y., Ohbayashi, N., Ohata, H., Mimata, A., Kukimoto-Niino, M., Moriyama, H., Kashimoto, S., Inoue, T., Goto, N., Okamoto, K., Shirouzu, M., Sawa, M., Yamada, T.(2016) Nat Commun 7: 12586-12586
- PubMed: 27562646
- DOI: https://doi.org/10.1038/ncomms12586
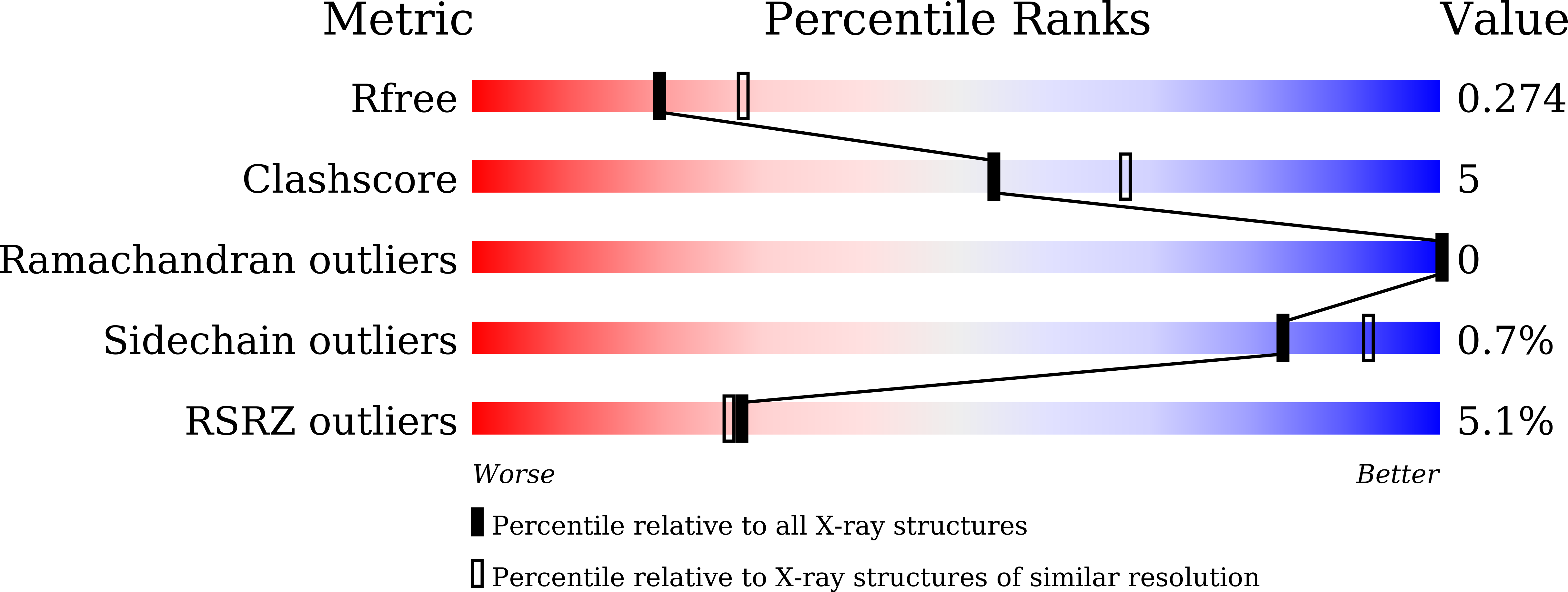
- Primary Citation of Related Structures:
5AX9, 5CWZ, 5D7A - PubMed Abstract:
Canonical Wnt/β-catenin signalling is essential for maintaining intestinal stem cells, and its constitutive activation has been implicated in colorectal carcinogenesis. We and others have previously identified Traf2- and Nck-interacting kinase (TNIK) as an essential regulatory component of the T-cell factor-4 and β-catenin transcriptional complex. Consistent with this, Tnik-deficient mice are resistant to azoxymethane-induced colon tumorigenesis, and Tnik(-/-)/Apc(min/+) mutant mice develop significantly fewer intestinal tumours. Here we report the first orally available small-molecule TNIK inhibitor, NCB-0846, having anti-Wnt activity. X-ray co-crystal structure analysis reveals that NCB-0846 binds to TNIK in an inactive conformation, and this binding mode seems to be essential for Wnt inhibition. NCB-0846 suppresses Wnt-driven intestinal tumorigenesis in Apc(min/+) mice and the sphere- and tumour-forming activities of colorectal cancer cells. TNIK is required for the tumour-initiating function of colorectal cancer stem cells. Its inhibition is a promising therapeutic approach.
Organizational Affiliation:
Division of Chemotherapy and Clinical Research, National Cancer Center Research Institute, 5-1-1 Tsukiji, Chuo-ku, Tokyo 104-0045, Japan.