Structural and Functional Insights Into tRNA Binding and Adenosine N1-Methylation by an Archaeal Trm10 Homologue.
Van Laer, B., Roovers, M., Wauters, L., Kasprzak, J.M., Dyzma, M., Deyaert, E., Kumar Singh, R., Feller, A., Bujnicki, J.M., Droogmans, L., Versees, W.(2016) Nucleic Acids Res 44: 940
- PubMed: 26673726
- DOI: https://doi.org/10.1093/nar/gkv1369
- Primary Citation of Related Structures:
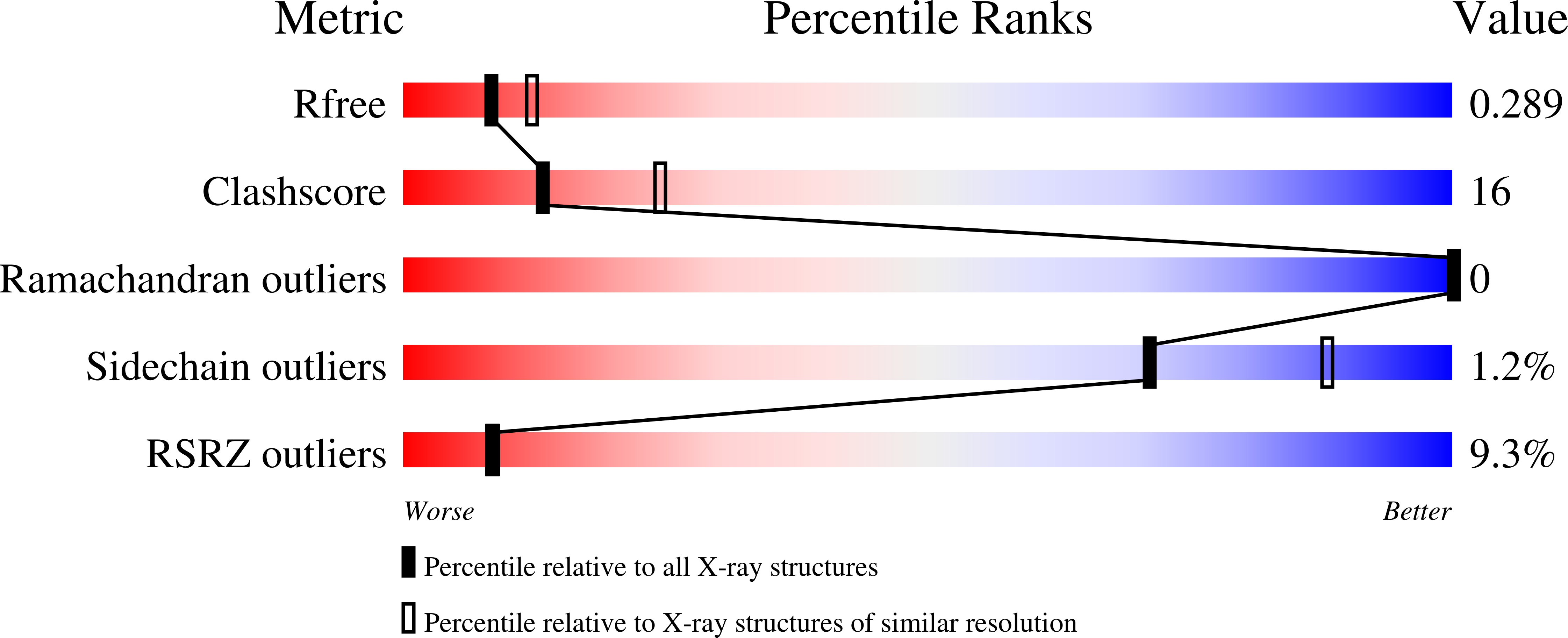
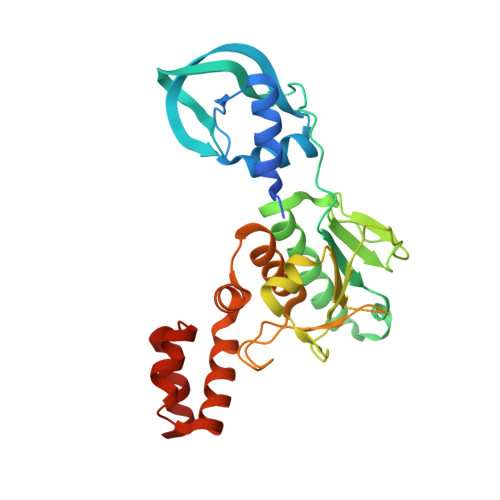
5A7T, 5A7Y, 5A7Z - PubMed Abstract:
Purine nucleosides on position 9 of eukaryal and archaeal tRNAs are frequently modified in vivo by the post-transcriptional addition of a methyl group on their N1 atom. The methyltransferase Trm10 is responsible for this modification in both these domains of life. While certain Trm10 orthologues specifically methylate either guanosine or adenosine at position 9 of tRNA, others have a dual specificity. Until now structural information about this enzyme family was only available for the catalytic SPOUT domain of Trm10 proteins that show specificity toward guanosine. Here, we present the first crystal structure of a full length Trm10 orthologue specific for adenosine, revealing next to the catalytic SPOUT domain also N- and C-terminal domains. This structure hence provides crucial insights in the tRNA binding mechanism of this unique monomeric family of SPOUT methyltransferases. Moreover, structural comparison of this adenosine-specific Trm10 orthologue with guanosine-specific Trm10 orthologues suggests that the N1 methylation of adenosine relies on additional catalytic residues.
Organizational Affiliation:
Structural Biology Brussels, Vrije Universiteit Brussel, Pleinlaan 2, 1050 Brussel, Belgium Structural Biology Research Center, VIB, Pleinlaan 2, 1050 Brussel, Belgium.