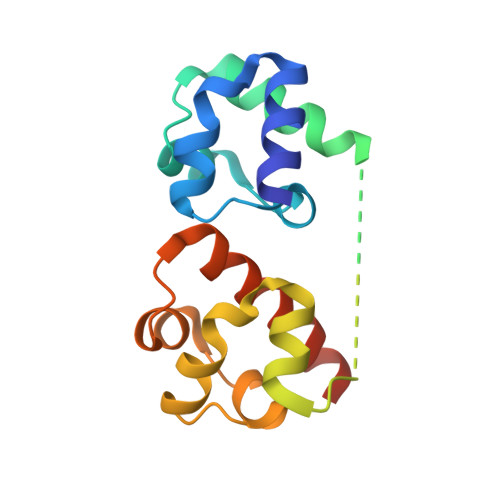
Specific Eph receptor-cytoplasmic effector signaling mediated by SAM-SAM domain interactions.
Wang, Y., Shang, Y., Li, J., Chen, W., Li, G., Wan, J., Liu, W., Zhang, M.(2018) Elife 7
- PubMed: 29749928
- DOI: https://doi.org/10.7554/eLife.35677
- Primary Citation of Related Structures:
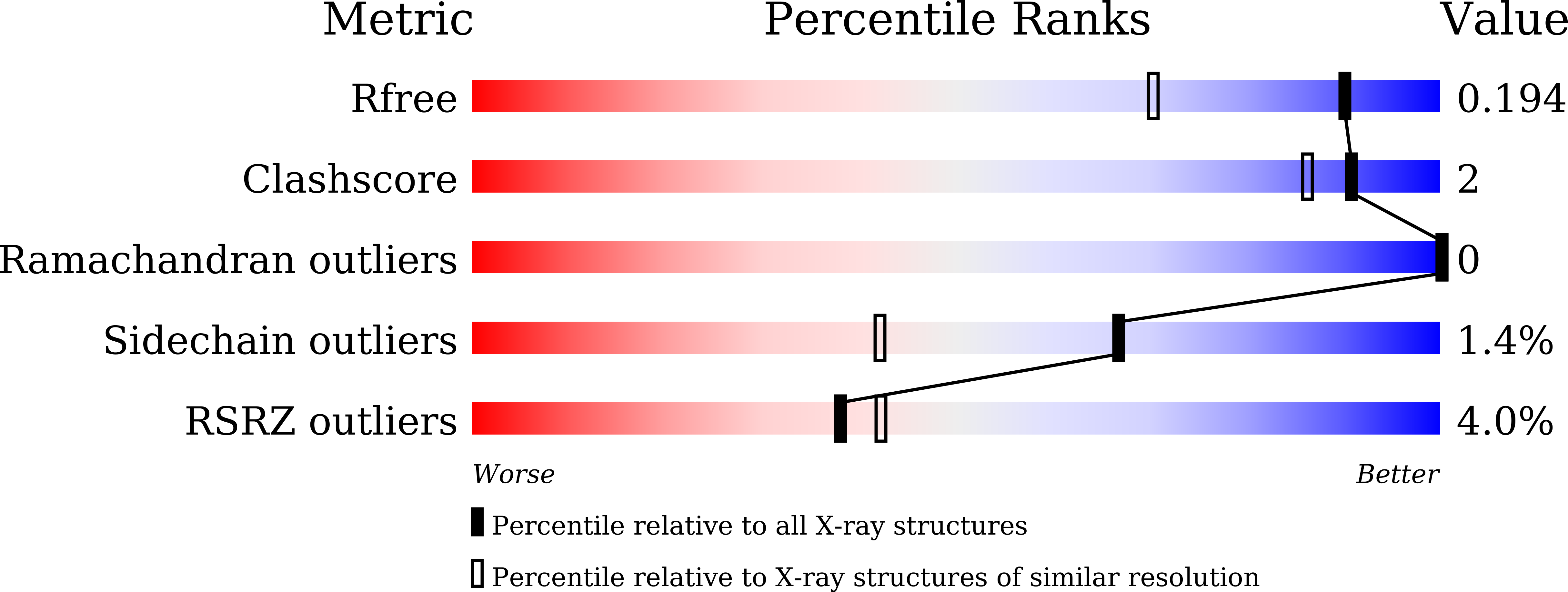
5ZRX, 5ZRY, 5ZRZ - PubMed Abstract:
The Eph receptor tyrosine kinase (RTK) family is the largest subfamily of RTKs playing critical roles in many developmental processes such as tissue patterning, neurogenesis and neuronal circuit formation, angiogenesis, etc. How the 14 Eph proteins, via their highly similar cytoplasmic domains, can transmit diverse and sometimes opposite cellular signals upon engaging ephrins is a major unresolved question. Here, we systematically investigated the bindings of each SAM domain of Eph receptors to the SAM domains from SHIP2 and Odin, and uncover a highly specific SAM-SAM interaction-mediated cytoplasmic Eph-effector binding pattern. Comparative X-ray crystallographic studies of several SAM-SAM heterodimer complexes, together with biochemical and cell biology experiments, not only revealed the exquisite specificity code governing Eph/effector interactions but also allowed us to identify SAMD5 as a new Eph binding partner. Finally, these Eph/effector SAM heterodimer structures can explain many Eph SAM mutations identified in patients suffering from cancers and other diseases.
Organizational Affiliation:
Shenzhen Key Laboratory for Neuronal Structural Biology, Biomedical Research Institute, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, China.