Structural and functional characterization of the transcriptional regulator Rv3488 ofMycobacterium tuberculosisH37Rv.
Kumari, M., Pal, R.K., Mishra, A.K., Tripathi, S., Biswal, B.K., Srivastava, K.K., Arora, A.(2018) Biochem J 475: 3393-3416
- PubMed: 30266832
- DOI: https://doi.org/10.1042/BCJ20180356
- Primary Citation of Related Structures:
5ZHC, 5ZHV, 5ZI8 - PubMed Abstract:
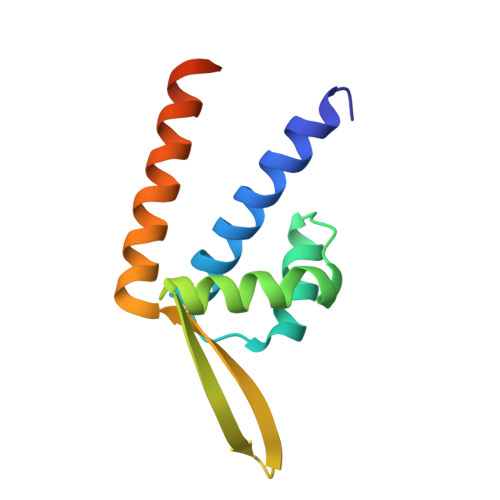
Rv3488 of Mycobacterium tuberculosis H37Rv has been assigned to the phenolic acid decarboxylase repressor (PadR) family of transcriptional regulators that play key roles in multidrug resistance and virulence of prokaryotes. The binding of cadmium, zinc, and several other metals to Rv3488 was discovered and characterized by isothermal titration calorimetery to be an exothermic process. Crystal structures of apo-Rv3488 and Rv3488 in complex with cadmium or zinc ions were determined by X-ray crystallography. The structure of Rv3488 revealed a dimeric protein with N-terminal winged-helix-turn-helix DNA-binding domains composed of helices α1, α2, α3, and strands β1 and β2, with the dimerization interface being formed of helices α4 and α1. The overall fold of Rv3488 was similar to PadR-s2 and metal sensor transcriptional regulators. In the crystal structure of Rv3488-Cd complex, two octahedrally coordinated Cd 2+ ions were present, one for each subunit. The same sites were occupied by zinc ions in the structure of Rv3488-Zn, with two additional zinc ions complexed in one monomer. EMSA studies showed specific binding of Rv3488 with its own 30-bp promoter DNA. The functional role of Rv3488 was characterized by expressing the rv3488 gene under the control of hsp60 promoter in Mycobacterium smegmatis Expression of Rv3488 increased the intracellular survival of recombinant M. smegmatis in murine macrophage cell line J774A.1 and also augmented its tolerance to Cd 2+ ions. Overall, the studies show that Rv3488 may have transcription regulation and metal-detoxifying functions and its expression in M. smegmatis increases intracellular survival, perhaps by counteracting toxic metal stress.
Organizational Affiliation:
Molecular and Structural Biology Division, CSIR-Central Drug Research Institute, Lucknow 226031, India.