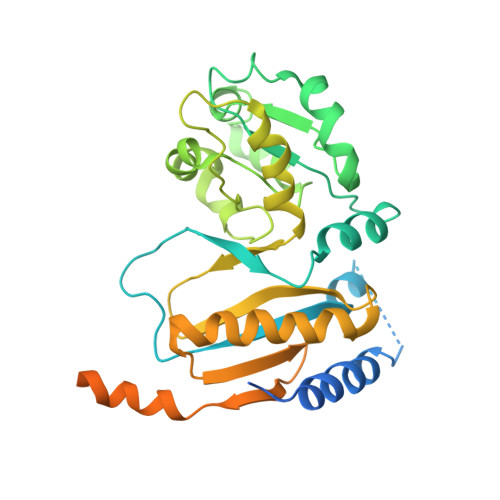
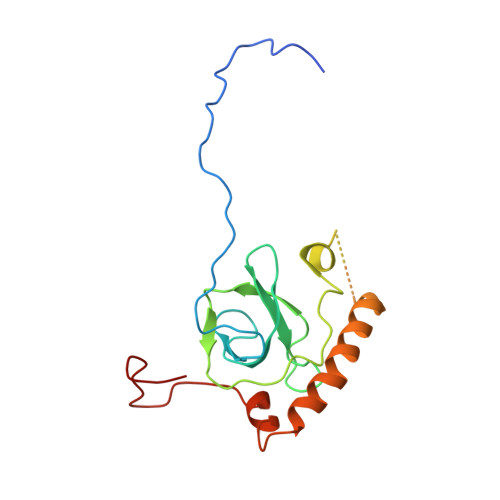
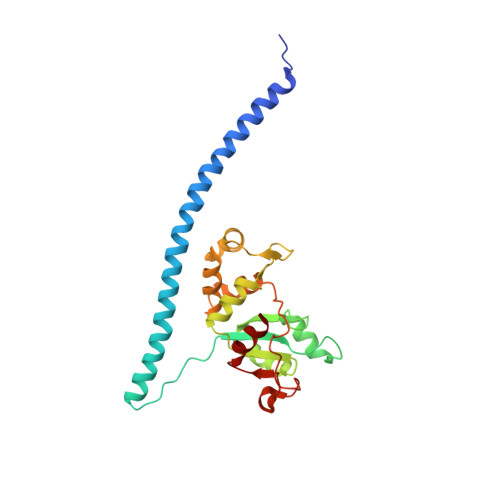
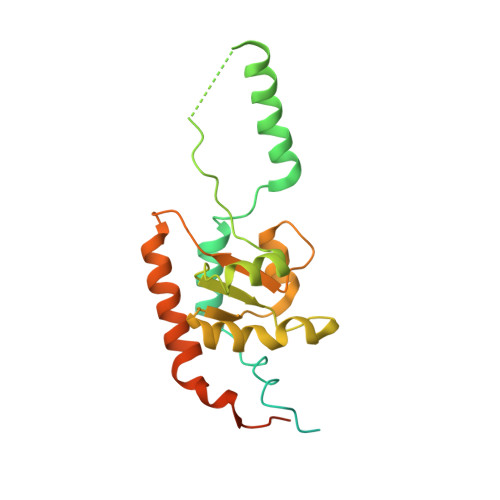
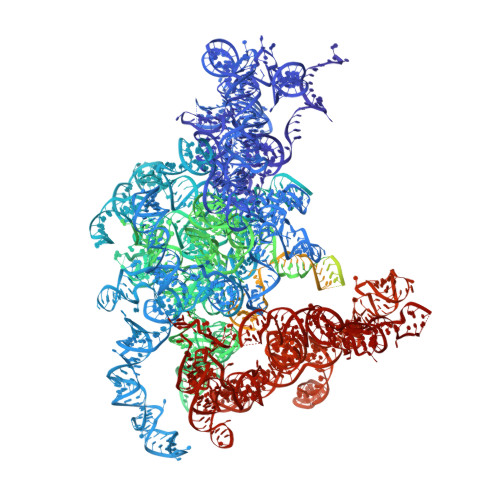
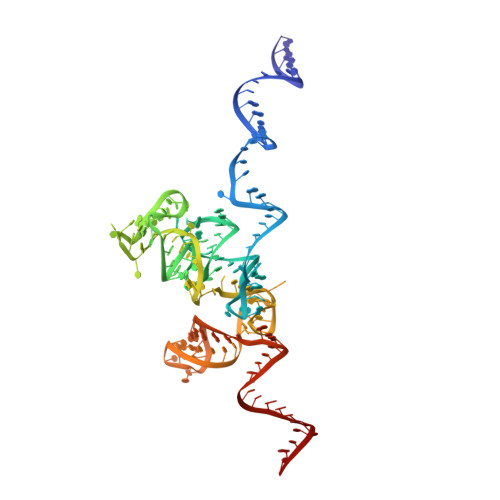
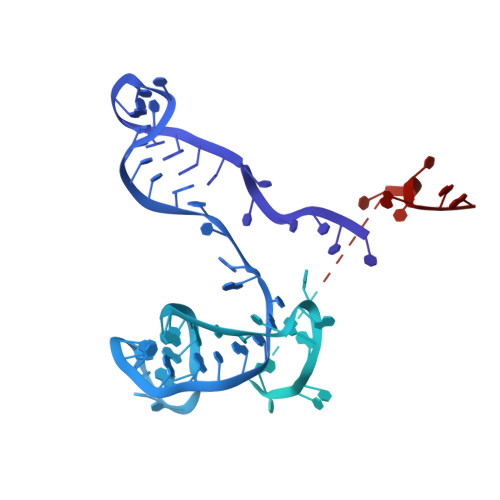
Cryo-EM structure of an early precursor of large ribosomal subunit reveals a half-assembled intermediate.
Zhou, D., Zhu, X., Zheng, S., Tan, D., Dong, M.Q., Ye, K.(2019) Protein Cell 10: 120-130
- PubMed: 29557065
- DOI: https://doi.org/10.1007/s13238-018-0526-7
- Primary Citation of Related Structures:
5Z1G, 5Z3G - PubMed Abstract:
Assembly of eukaryotic ribosome is a complicated and dynamic process that involves a series of intermediates. It is unknown how the highly intertwined structure of 60S large ribosomal subunits is established. Here, we report the structure of an early nucleolar pre-60S ribosome determined by cryo-electron microscopy at 3.7 Å resolution, revealing a half-assembled subunit. Domains I, II and VI of 25S/5.8S rRNA pack tightly into a native-like substructure, but domains III, IV and V are not assembled. The structure contains 12 assembly factors and 19 ribosomal proteins, many of which are required for early processing of large subunit rRNA. The Brx1-Ebp2 complex would interfere with the assembly of domains IV and V. Rpf1, Mak16, Nsa1 and Rrp1 form a cluster that consolidates the joining of domains I and II. Our structure reveals a key intermediate on the path to establishing the global architecture of 60S subunits.
Organizational Affiliation:
Graduate School of Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, 100730, China.