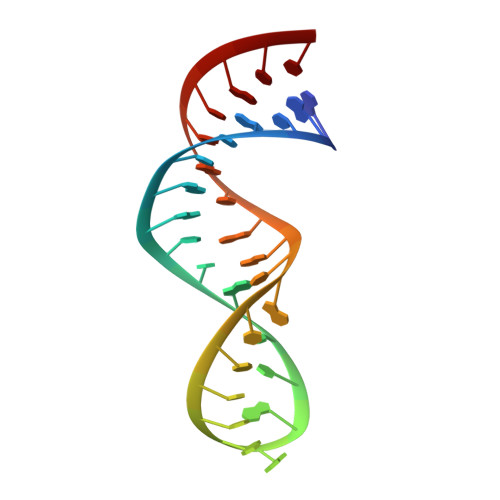
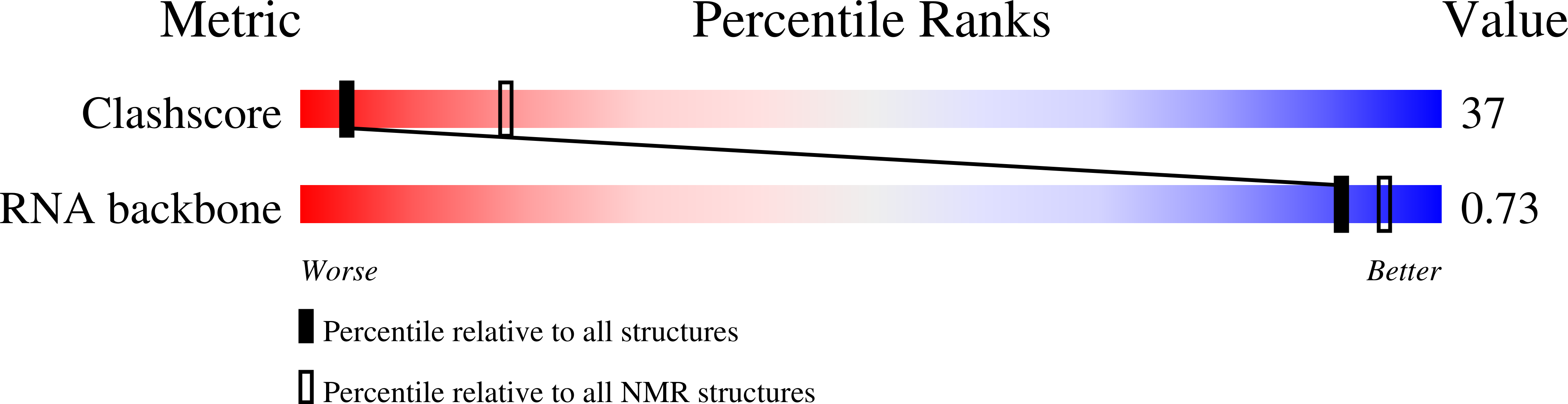A Macrocyclic Peptide Ligand Binds the Oncogenic MicroRNA-21 Precursor and Suppresses Dicer Processing.
Shortridge, M.D., Walker, M.J., Pavelitz, T., Chen, Y., Yang, W., Varani, G.(2017) ACS Chem Biol 12: 1611-1620
- PubMed: 28437065
- DOI: https://doi.org/10.1021/acschembio.7b00180
- Primary Citation of Related Structures:
5UZT, 5UZZ - PubMed Abstract:
MicroRNAs (miRNAs) help orchestrate cellular growth and survival through post-transcriptional mechanisms. The dysregulation of miRNA biogenesis can lead to cellular growth defects and chemotherapeutic resistance and plays a direct role in the development of many chronic diseases. Among these RNAs, miR-21 is consistently overexpressed in most human cancers, leading to the down-regulation of key tumor-suppressing and pro-apoptotic factors, suggesting that inhibition of miR-21 biogenesis could reverse these negative effects. However, targeted inhibition of miR-21 using small molecules has had limited success. To overcome difficulties in targeting RNA secondary structure with small molecules, we developed a class of cyclic β-hairpin peptidomimetics which bind to RNA stem-loop structures, such as miRNA precursors, with potent affinity and specificity. We screened an existing cyclic peptide library and discovered a lead structure which binds to pre-miR21 with K D = 200 nM and prefers it over other pre-miRNAs. The NMR structure of the complex shows that the peptide recognizes the Dicer cleavage site and alters processing of the precursor to the mature miRNA in vitro and in cultured cells. The structure provides a rationale for the peptide binding activity and clear guidance for further improvements in affinity and targeting.
Organizational Affiliation:
Department of Chemistry, University of Washington, Seattle , Box 351700, Seattle, Washington 98195, United States.