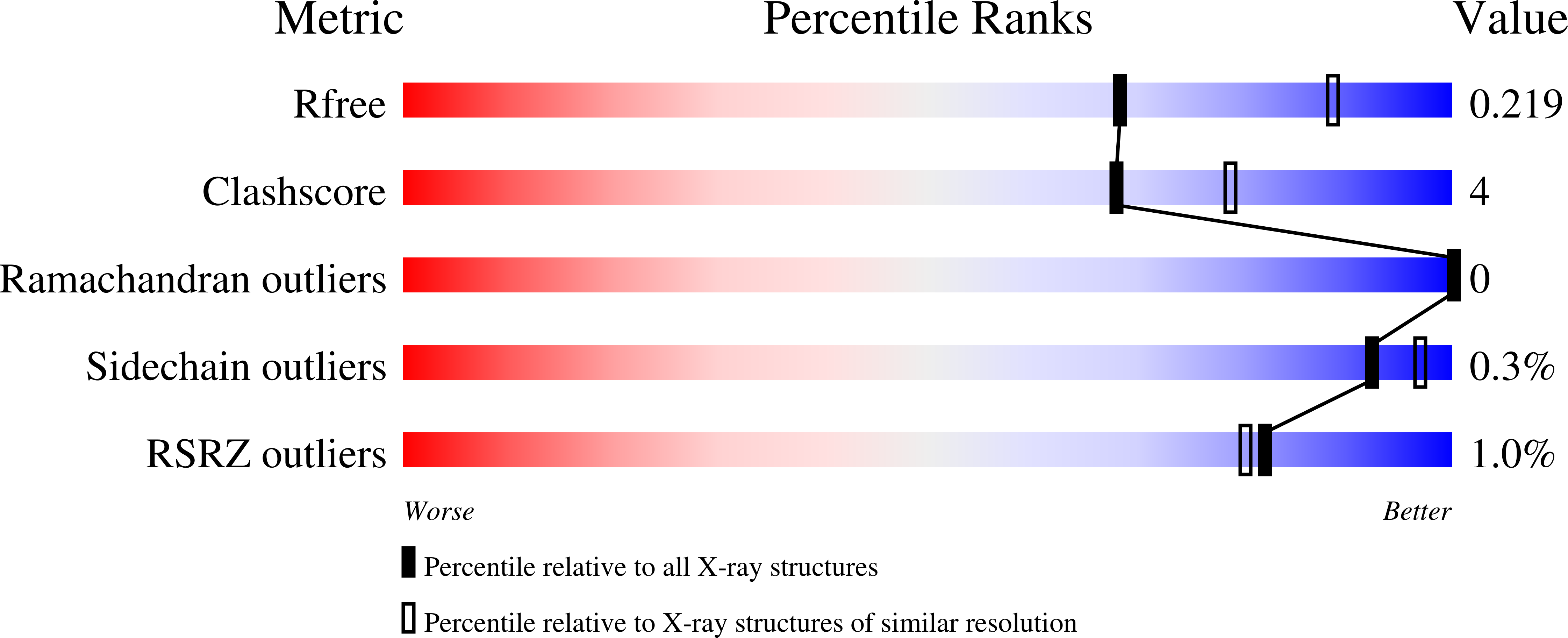
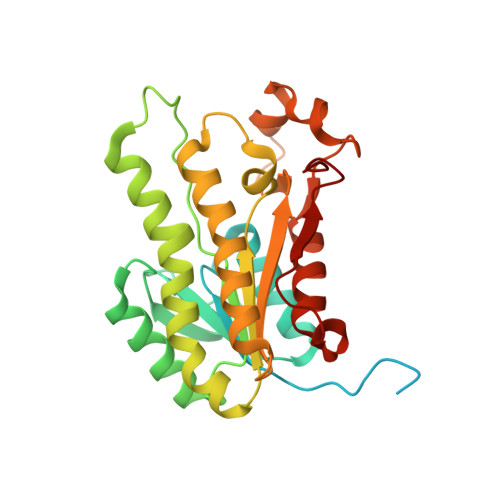
Structural rearrangements occurring upon cofactor binding in the Mycobacterium smegmatis beta-ketoacyl-acyl carrier protein reductase MabA.
Kussau, T., Flipo, M., Van Wyk, N., Viljoen, A., Olieric, V., Kremer, L., Blaise, M.(2018) Acta Crystallogr D Struct Biol 74: 383-393
- PubMed: 29717709
- DOI: https://doi.org/10.1107/S2059798318002917
- Primary Citation of Related Structures:
5OVJ, 5OVK, 5OVL - PubMed Abstract:
In mycobacteria, the ketoacyl-acyl carrier protein (ACP) reductase MabA (designated FabG in other bacteria) catalyzes the NADPH-dependent reduction of β-ketoacyl-ACP substrates to β-hydroxyacyl-ACP products. This first reductive step in the fatty-acid biosynthesis elongation cycle is essential for bacteria, which makes MabA/FabG an interesting drug target. To date, however, very few molecules targeting FabG have been discovered and MabA remains the only enzyme of the mycobacterial type II fatty-acid synthase that lacks specific inhibitors. Despite the existence of several MabA/FabG crystal structures, the structural rearrangement that occurs upon cofactor binding is still not fully understood. Therefore, unlocking this knowledge gap could help in the design of new inhibitors. Here, high-resolution crystal structures of MabA from Mycobacterium smegmatis in its apo, NADP + -bound and NADPH-bound forms are reported. Comparison of these crystal structures reveals the structural reorganization of the lid region covering the active site of the enzyme. The crystal structure of the apo form revealed numerous residues that trigger steric hindrance to the binding of NADPH and substrate. Upon NADPH binding, these residues are pushed away from the active site, allowing the enzyme to adopt an open conformation. The transition from an NADPH-bound to an NADP + -bound form is likely to facilitate release of the product. These results may be useful for subsequent rational drug design and/or for in silico drug-screening approaches targeting MabA/FabG.
Organizational Affiliation:
Institut de Recherche en Infectiologie de Montpellier (IRIM), Université de Montpellier, CNRS UMR 9004, 34293 Montpellier, France.