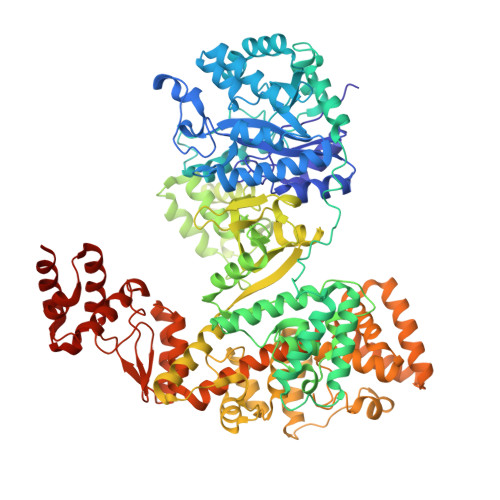
High-fidelity DNA replication in Mycobacterium tuberculosis relies on a trinuclear zinc center.
Banos-Mateos, S., van Roon, A.M., Lang, U.F., Maslen, S.L., Skehel, J.M., Lamers, M.H.(2017) Nat Commun 8: 855-855
- PubMed: 29021523
- DOI: https://doi.org/10.1038/s41467-017-00886-w
- Primary Citation of Related Structures:
5LEW - PubMed Abstract:
High-fidelity DNA replication depends on a proofreading 3'-5' exonuclease that is associated with the replicative DNA polymerase. The replicative DNA polymerase DnaE1 from the major pathogen Mycobacterium tuberculosis (Mtb) uses its intrinsic PHP-exonuclease that is distinct from the canonical DEDD exonucleases found in the Escherichia coli and eukaryotic replisomes. The mechanism of the PHP-exonuclease is not known. Here, we present the crystal structure of the Mtb DnaE1 polymerase. The PHP-exonuclease has a trinuclear zinc center, coordinated by nine conserved residues. Cryo-EM analysis reveals the entry path of the primer strand in the PHP-exonuclease active site. Furthermore, the PHP-exonuclease shows a striking similarity to E. coli endonuclease IV, which provides clues regarding the mechanism of action. Altogether, this work provides important insights into the PHP-exonuclease and reveals unique properties that make it an attractive target for novel anti-mycobacterial drugs.The polymerase and histidinol phosphatase (PHP) domain in the DNA polymerase DnaE1 is essential for mycobacterial high-fidelity DNA replication. Here, the authors determine the DnaE1 crystal structure, which reveals the PHP-exonuclease mechanism that can be exploited for antibiotic development.
Organizational Affiliation:
MRC Laboratory of Molecular Biology, Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, CB2 0QH, UK.