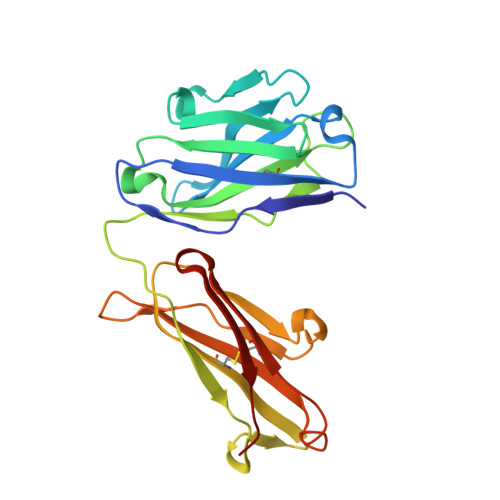
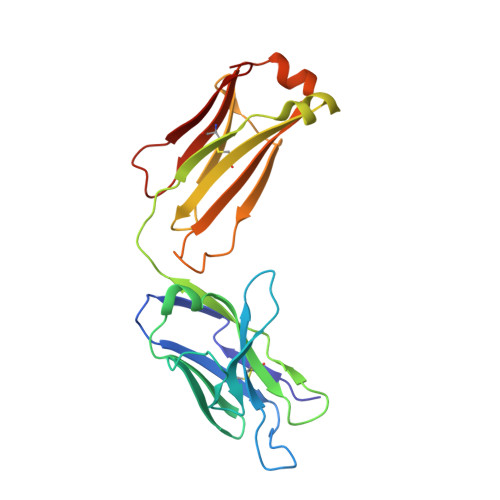
Structure-guided residence time optimization of a dabigatran reversal agent.
Schiele, F., van Ryn, J., Litzenburger, T., Ritter, M., Seeliger, D., Nar, H.(2015) MAbs 7: 871-880
- PubMed: 26047352
- DOI: https://doi.org/10.1080/19420862.2015.1057364
- Primary Citation of Related Structures:
4YGV, 4YHI, 4YHK, 4YHL, 4YHM, 4YHN, 4YHO - PubMed Abstract:
Novel oral anticoagulants are effective and safe alternatives to vitamin-K antagonists for anticoagulation therapy. However, anticoagulation therapy in general is associated with an elevated risk of bleeding. Idarucizumab is a reversal agent for the direct thrombin inhibitor, dabigatran etexilate (Pradaxa®) and is currently in Phase 3 studies. Here, we report data on the antibody fragment aDabi-Fab2, a putative backup molecule for idarucizumab. Although aDabi-Fab2 completely reversed effects of dabigatran in a rat model in vivo, we observed significantly reduced duration of action compared to idarucizumab. Rational protein engineering, based on the X-ray structure of aDabi-Fab2, led to the identification of mutant Y103W. The mutant had optimized shape complementarity to dabigatran while maintaining an energetically favored hydrogen bond. It displayed increased affinity for dabigatran, mainly driven by a slower off-rate. Interestingly, the increased residence time translated into longer duration of action in vivo. It was thus possible to further enhance the efficacy of aDabi-Fab2 based on rational design, giving it the potential to serve as a back-up candidate for idarucizumab.
Organizational Affiliation:
a New Biological Entities Discovery; Boehringer Ingelheim GmbH & Co. KG ; Biberach , Germany.