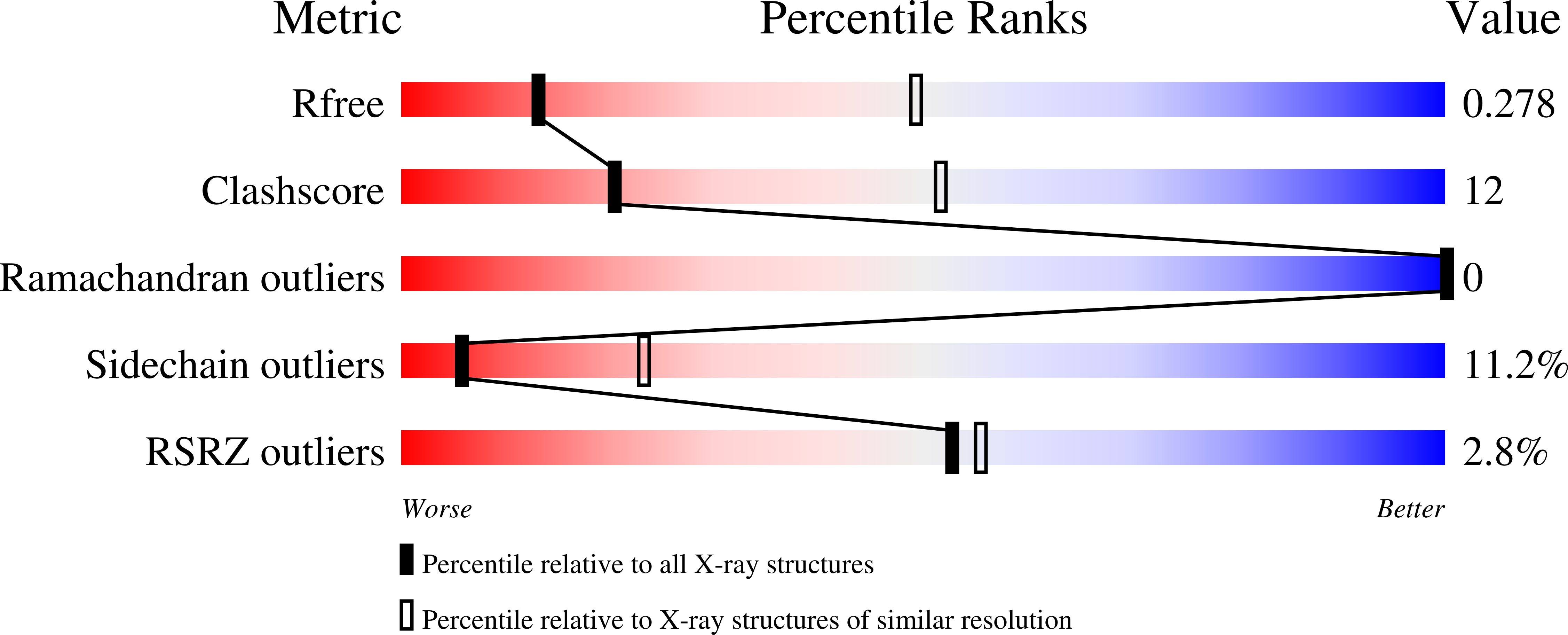
HU multimerization shift controls nucleoid compaction.
Hammel, M., Amlanjyoti, D., Reyes, F.E., Chen, J.H., Parpana, R., Tang, H.Y., Larabell, C.A., Tainer, J.A., Adhya, S.(2016) Sci Adv 2: e1600650-e1600650
- PubMed: 27482541
- DOI: https://doi.org/10.1126/sciadv.1600650
- Primary Citation of Related Structures:
4YEW, 4YEX, 4YEY, 4YF0, 4YFH, 4YFT - PubMed Abstract:
Molecular mechanisms controlling functional bacterial chromosome (nucleoid) compaction and organization are surprisingly enigmatic but partly depend on conserved, histone-like proteins HUαα and HUαβ and their interactions that span the nanoscale and mesoscale from protein-DNA complexes to the bacterial chromosome and nucleoid structure. We determined the crystal structures of these chromosome-associated proteins in complex with native duplex DNA. Distinct DNA binding modes of HUαα and HUαβ elucidate fundamental features of bacterial chromosome packing that regulate gene transcription. By combining crystal structures with solution x-ray scattering results, we determined architectures of HU-DNA nucleoproteins in solution under near-physiological conditions. These macromolecular conformations and interactions result in contraction at the cellular level based on in vivo imaging of native unlabeled nucleoid by soft x-ray tomography upon HUβ and ectopic HUα38 expression. Structural characterization of charge-altered HUαα-DNA complexes reveals an HU molecular switch that is suitable for condensing nucleoid and reprogramming noninvasive Escherichia coli into an invasive form. Collective findings suggest that shifts between networking and cooperative and noncooperative DNA-dependent HU multimerization control DNA compaction and supercoiling independently of cellular topoisomerase activity. By integrating x-ray crystal structures, x-ray scattering, mutational tests, and x-ray imaging that span from protein-DNA complexes to the bacterial chromosome and nucleoid structure, we show that defined dynamic HU interaction networks can promote nucleoid reorganization and transcriptional regulation as efficient general microbial mechanisms to help synchronize genetic responses to cell cycle, changing environments, and pathogenesis.
Organizational Affiliation:
Molecular Biophysics and Integrated Bioimaging, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA.