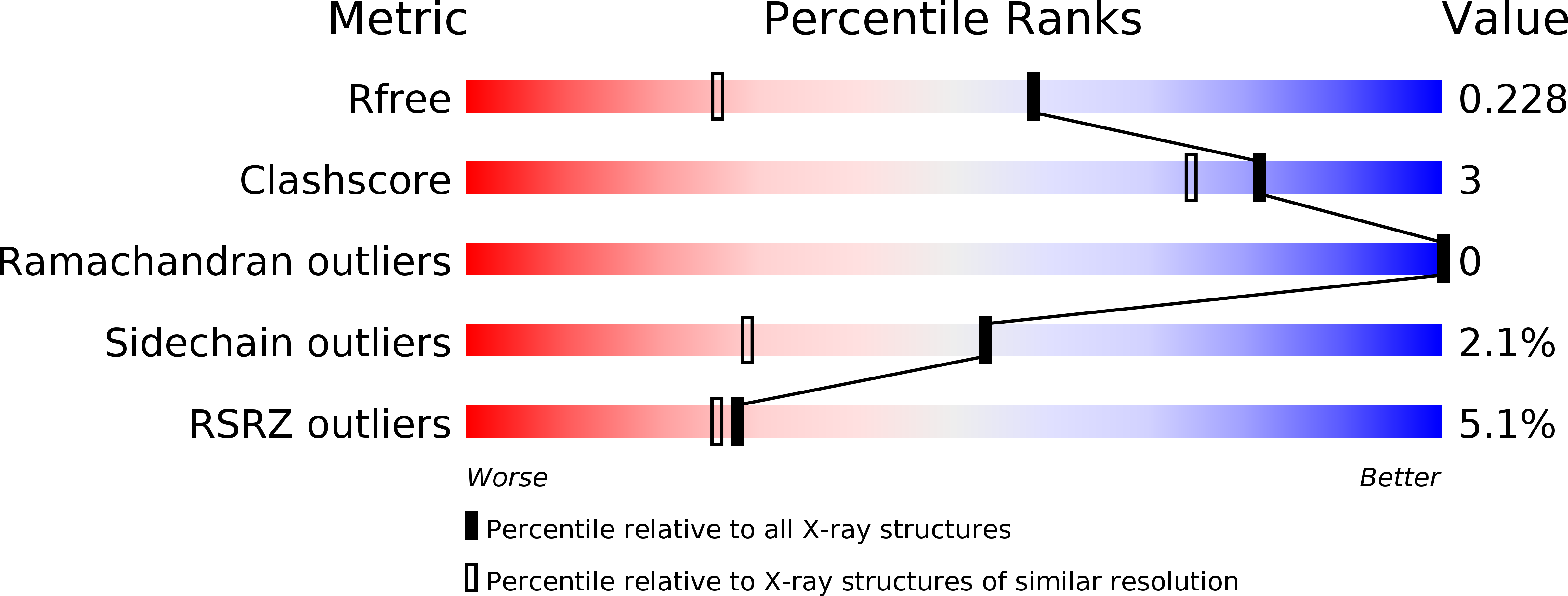
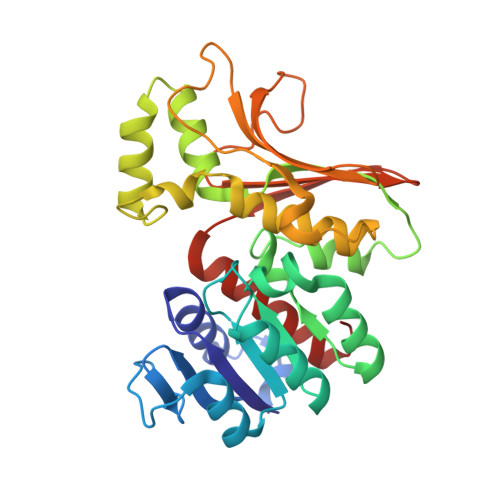
Structural insight into activation of homoserine dehydrogenase from the archaeonSulfolobus tokodaiivia reduction.
Tomonaga, Y., Kaneko, R., Goto, M., Ohshima, T., Yoshimune, K.(2015) Biochem Biophys Rep 3: 14-17
- PubMed: 29124164
- DOI: https://doi.org/10.1016/j.bbrep.2015.07.006
- Primary Citation of Related Structures:
4YDR, 5AVO - PubMed Abstract:
Homoserine dehydrogenase (HSD; 305 amino acid residues) catalyzes an NAD(P)-dependent reversible reaction between l-homoserine and aspartate 4-semialdehyde and is involved in the aspartate pathway. HSD from the hyperthermophilic archaeon Sulfolobus tokodaii was markedly activated (2.5-fold) by the addition of 0.8 mM dithiothreitol. The crystal structure of the homodimer indicated that the activation was caused by cleavage of the disulfide bond formed between two cysteine residues (C303) in the C-terminal regions of the two subunits.
Organizational Affiliation:
Department of Applied Molecular Chemistry, Graduate School of Industrial Technology, Nihon University, 1-2-1, Izumichou, Narashino, Chiba 275-8575, Japan.