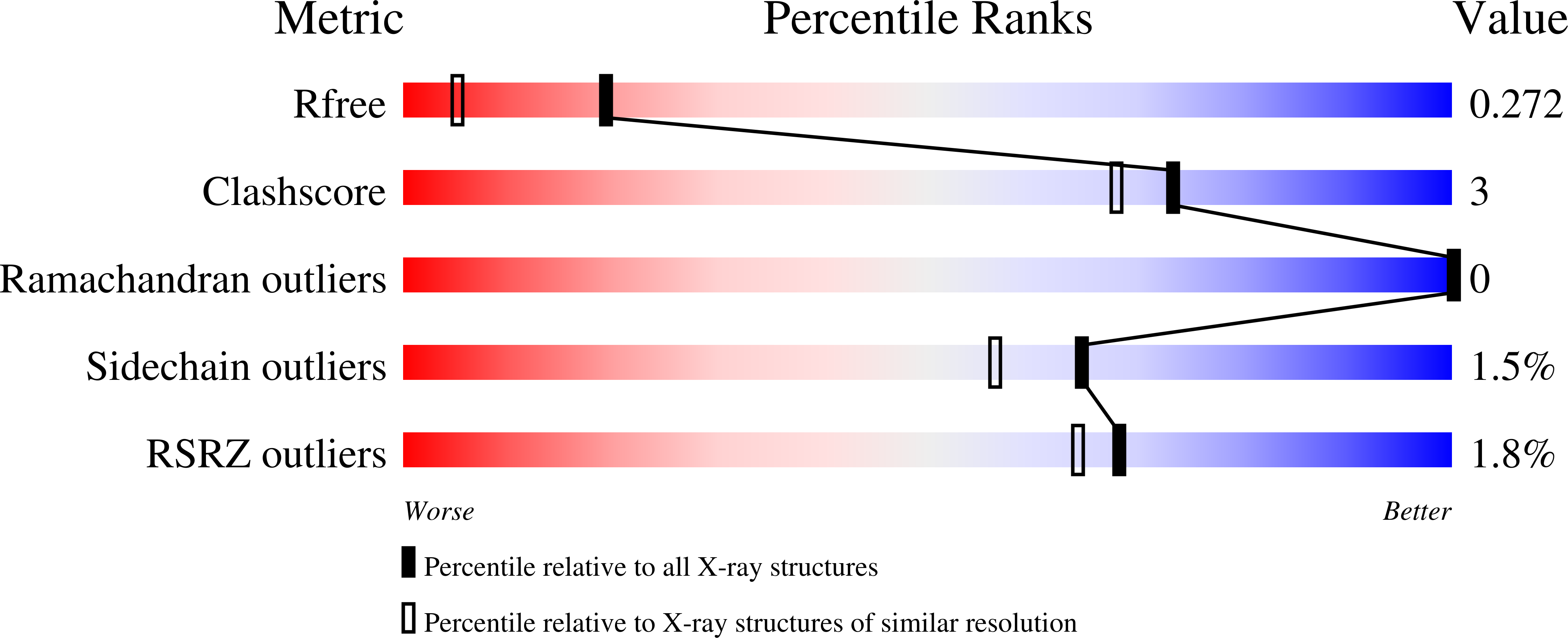
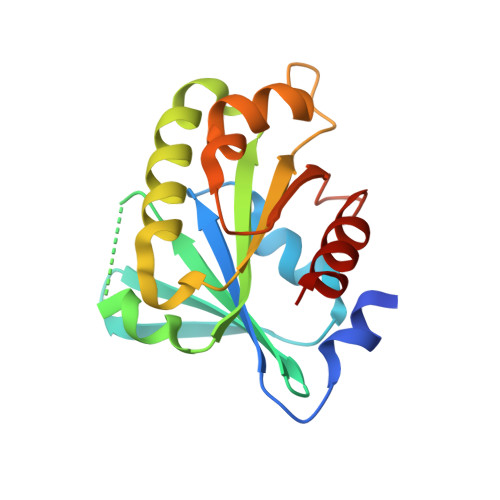
Structure of an ADP-ribosylation factor, ARF1, from Entamoeba histolytica bound to Mg(2+)-GDP.
Serbzhinskiy, D.A., Clifton, M.C., Sankaran, B., Staker, B.L., Edwards, T.E., Myler, P.J.(2015) Acta Crystallogr F Struct Biol Commun 71: 594-599
- PubMed: 25945714
- DOI: https://doi.org/10.1107/S2053230X15004677
- Primary Citation of Related Structures:
4Y0V, 4YLG - PubMed Abstract:
Entamoeba histolytica is the etiological agent of amebiasis, a diarrheal disease which causes amoebic liver abscesses and amoebic colitis. Approximately 50 million people are infected worldwide with E. histolytica. With only 10% of infected people developing symptomatic amebiasis, there are still an estimated 100,000 deaths each year. Because of the emergence of resistant strains of the parasite, it is necessary to find a treatment which would be a proper response to this challenge. ADP-ribosylation factor (ARF) is a member of the ARF family of GTP-binding proteins. These proteins are ubiquitous in eukaryotic cells; they generally associate with cell membranes and regulate vesicular traffic and intracellular signalling. The crystal structure of ARF1 from E. histolytica has been determined bound to magnesium and GDP at 1.8 Å resolution. Comparison with other structures of eukaryotic ARF proteins shows a highly conserved structure and supports the interswitch toggle mechanism of communicating the conformational state to partner proteins.
Organizational Affiliation:
Seattle Structural Genomics Center for Infectious Disease (SSGCID), USA.