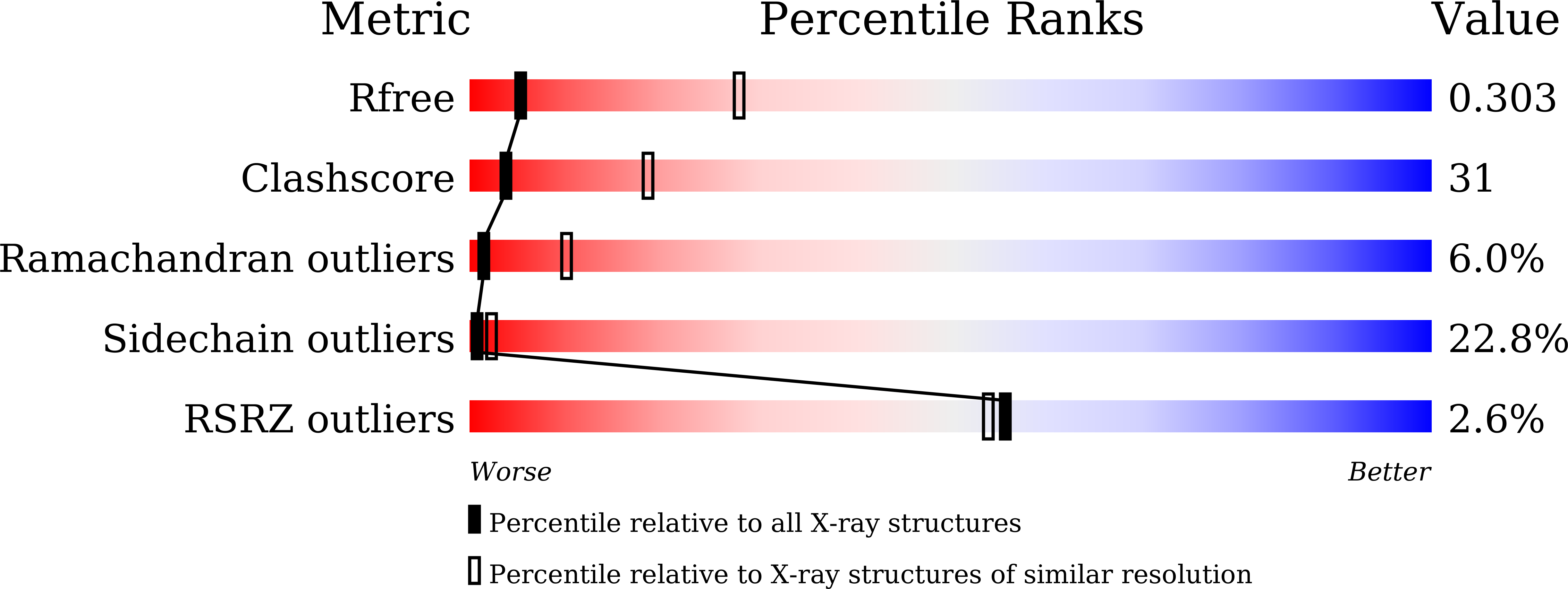
Crystal Structure of Apo and Ligand Bound Vibrio choleraeRibokinase (Vc-RK): Role of Monovalent Cation Induced Activation and Structural Flexibility in Sugar Phosphorylation
Paul, R., Patra, M.D., Sen, U.(2015) Adv Exp Med Biol 842: 293-307
- PubMed: 25408351
- DOI: https://doi.org/10.1007/978-3-319-11280-0_19
- Primary Citation of Related Structures:
4X8F, 4XCK, 4XDA
Organizational Affiliation:
Crystallography and Molecular Biology Division, Saha Institute of Nuclear Physics, 1/AF Bidhan Nagar, WB, Kolkata, 700 064, India.