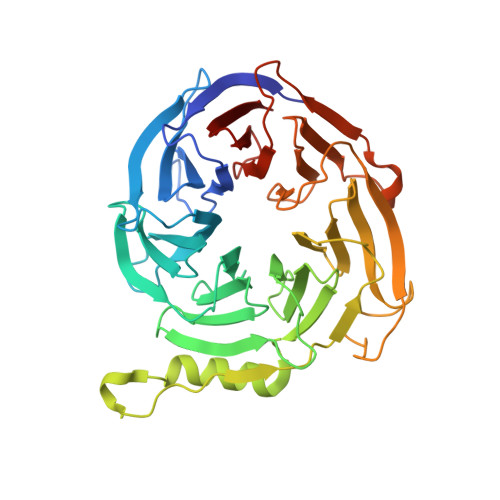
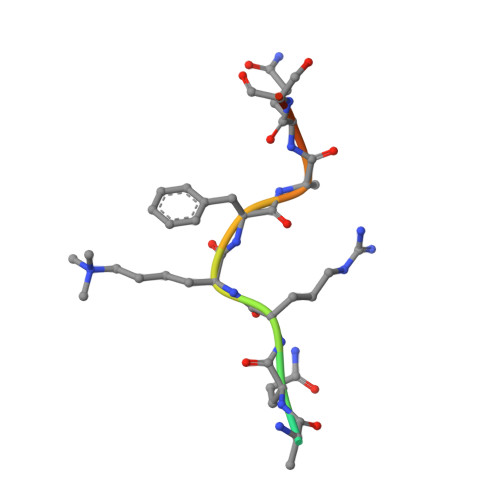
Jarid2 Methylation via the PRC2 Complex Regulates H3K27me3 Deposition during Cell Differentiation.
Sanulli, S., Justin, N., Teissandier, A., Ancelin, K., Portoso, M., Caron, M., Michaud, A., Lombard, B., da Rocha, S.T., Offer, J., Loew, D., Servant, N., Wassef, M., Burlina, F., Gamblin, S.J., Heard, E., Margueron, R.(2015) Mol Cell 57: 769-783
- PubMed: 25620564
- DOI: https://doi.org/10.1016/j.molcel.2014.12.020
- Primary Citation of Related Structures:
4X3E - PubMed Abstract:
Polycomb Group (PcG) proteins maintain transcriptional repression throughout development, mostly by regulating chromatin structure. Polycomb Repressive Complex 2 (PRC2), a component of the Polycomb machinery, is responsible for the methylation of histone H3 lysine 27 (H3K27me2/3). Jarid2 was previously identified as a cofactor of PRC2, regulating PRC2 targeting to chromatin and its enzymatic activity. Deletion of Jarid2 leads to impaired orchestration of gene expression during cell lineage commitment. Here, we reveal an unexpected crosstalk between Jarid2 and PRC2, with Jarid2 being methylated by PRC2. This modification is recognized by the Eed core component of PRC2 and triggers an allosteric activation of PRC2's enzymatic activity. We show that Jarid2 methylation is important to promote PRC2 activity at a locus devoid of H3K27me3 and for the correct deposition of this mark during cell differentiation. Our results uncover a regulation loop where Jarid2 methylation fine-tunes PRC2 activity depending on the chromatin context.
Organizational Affiliation:
Institut Curie, 26 Rue d'Ulm, 75005 Paris, France; INSERM U934, 26 Rue d'Ulm, 75005 Paris, France; CNRS UMR3215, 26 Rue d'Ulm, 75005 Paris, France.