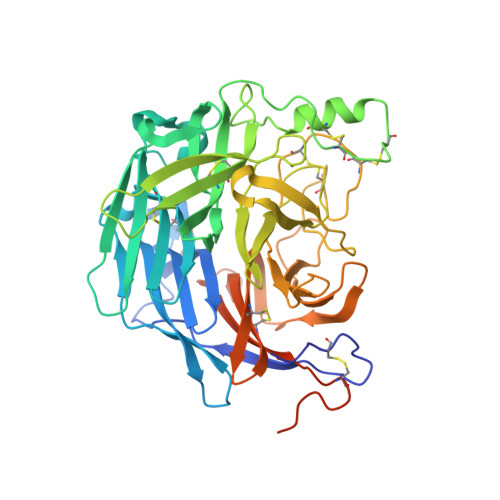
Molecular Recognition of Human Ephrinb2 Cell Surface Receptor by an Emergent African Henipavirus.
Lee, B., Pernet, O., Ahmed, A.A., Zeltina, A., Beaty, S.M., Bowden, T.A.(2015) Proc Natl Acad Sci U S A 112: E2156
- PubMed: 25825759
- DOI: https://doi.org/10.1073/pnas.1501690112
- Primary Citation of Related Structures:
4UF7 - PubMed Abstract:
The discovery of African henipaviruses (HNVs) related to pathogenic Hendra virus (HeV) and Nipah virus (NiV) from Southeast Asia and Australia presents an open-ended health risk. Cell receptor use by emerging African HNVs at the stage of host-cell entry is a key parameter when considering the potential for spillover and infection of human populations. The attachment glycoprotein from a Ghanaian bat isolate (GhV-G) exhibits <30% sequence identity with Asiatic NiV-G/HeV-G. Here, through functional and structural analysis of GhV-G, we show how this African HNV targets the same human cell-surface receptor (ephrinB2) as the Asiatic HNVs. We first characterized this virus-receptor interaction crystallographically. Compared with extant HNV-G-ephrinB2 structures, there was significant structural variation in the six-bladed β-propeller scaffold of the GhV-G receptor-binding domain, but not the Greek key fold of the bound ephrinB2. Analysis revealed a surprisingly conserved mode of ephrinB2 interaction that reflects an ongoing evolutionary constraint among geographically distal and phylogenetically divergent HNVs to maintain the functionality of ephrinB2 recognition during virus-host entry. Interestingly, unlike NiV-G/HeV-G, we could not detect binding of GhV-G to ephrinB3. Comparative structure-function analysis further revealed several distinguishing features of HNV-G function: a secondary ephrinB2 interaction site that contributes to more efficient ephrinB2-mediated entry in NiV-G relative to GhV-G and cognate residues at the very C terminus of GhV-G (absent in Asiatic HNV-Gs) that are vital for efficient receptor-induced fusion, but not receptor binding per se. These data provide molecular-level details for evaluating the likelihood of African HNVs to spill over into human populations.
Organizational Affiliation:
Department of Microbiology, Icahn School of Medicine at Mount Sinai, New York, NY 10029; thomas.bowden@strubi.ox.ac.uk benhur.lee@mssm.edu.