Crystal Structure of WW3 domain of ITCH in complex with TXNIP peptide
Liu, Y., Tempel, W., Bountra, C., Arrowsmith, C.H., Edwards, A.M., Min, J., Structural Genomics Consortium (SGC)To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| E3 ubiquitin-protein ligase Itchy homolog | 47 | Homo sapiens | Mutation(s): 0 Gene Names: ITCH EC: 6.3.2 | 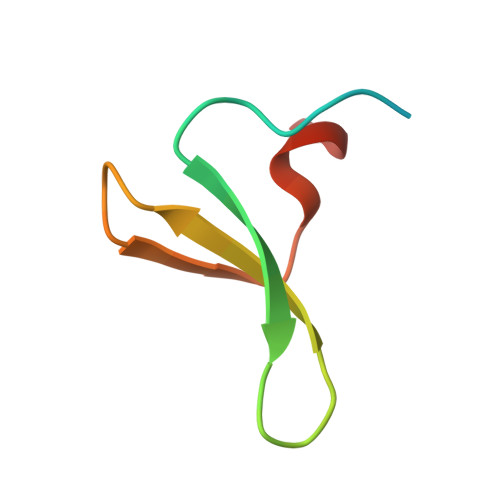 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96J02 (Homo sapiens) Explore Q96J02 Go to UniProtKB: Q96J02 | |||||
PHAROS: Q96J02 GTEx: ENSG00000078747 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96J02 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Thioredoxin-interacting protein | 14 | Homo sapiens | Mutation(s): 2 | 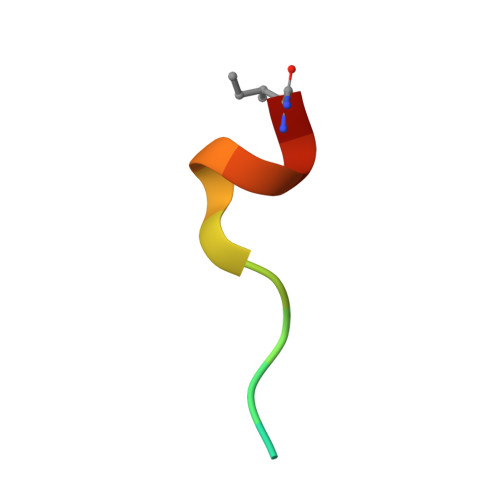 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9H3M7 (Homo sapiens) Explore Q9H3M7 Go to UniProtKB: Q9H3M7 | |||||
PHAROS: Q9H3M7 GTEx: ENSG00000265972 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9H3M7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 30.171 | α = 90 |
| b = 55.312 | β = 90 |
| c = 59.796 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| PHASER | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |