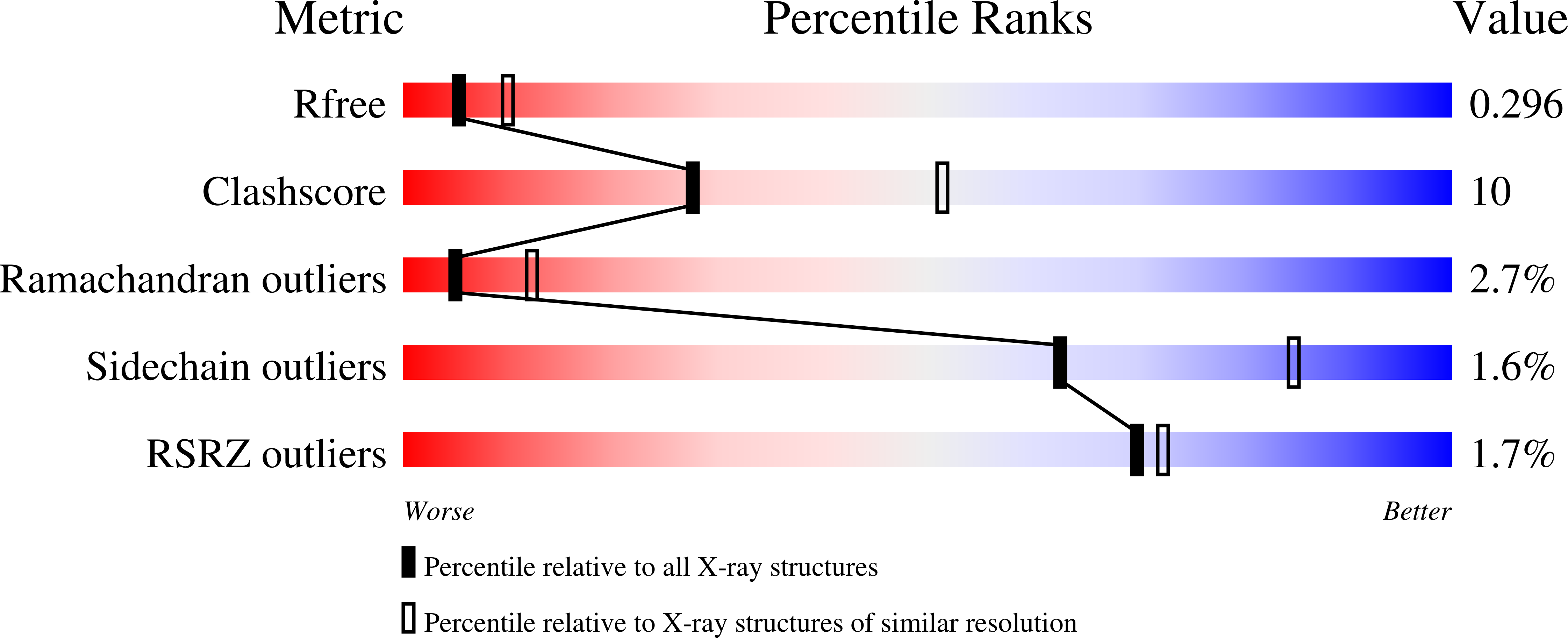
A structural, functional, and computational analysis suggests pore flexibility as the base for the poor selectivity of CNG channels.
Napolitano, L.M., Bisha, I., De March, M., Marchesi, A., Arcangeletti, M., Demitri, N., Mazzolini, M., Rodriguez, A., Magistrato, A., Onesti, S., Laio, A., Torre, V.(2015) Proc Natl Acad Sci U S A 112: E3619-E3628
- PubMed: 26100907
- DOI: https://doi.org/10.1073/pnas.1503334112
- Primary Citation of Related Structures:
4R50, 4R6Z, 4R7C, 4R8C, 4RAI, 4RO2 - PubMed Abstract:
Cyclic nucleotide-gated (CNG) ion channels, despite a significant homology with the highly selective K(+) channels, do not discriminate among monovalent alkali cations and are permeable also to several organic cations. We combined electrophysiology, molecular dynamics (MD) simulations, and X-ray crystallography to demonstrate that the pore of CNG channels is highly flexible. When a CNG mimic is crystallized in the presence of a variety of monovalent cations, including Na(+), Cs(+), and dimethylammonium (DMA(+)), the side chain of Glu66 in the selectivity filter shows multiple conformations and the diameter of the pore changes significantly. MD simulations indicate that Glu66 and the prolines in the outer vestibule undergo large fluctuations, which are modulated by the ionic species and the voltage. This flexibility underlies the coupling between gating and permeation and the poor ionic selectivity of CNG channels.
Organizational Affiliation:
Structural Biology Laboratory, Elettra-Sincrotrone Trieste S.C.p.A., Basovizza, Trieste 34149, Italy;