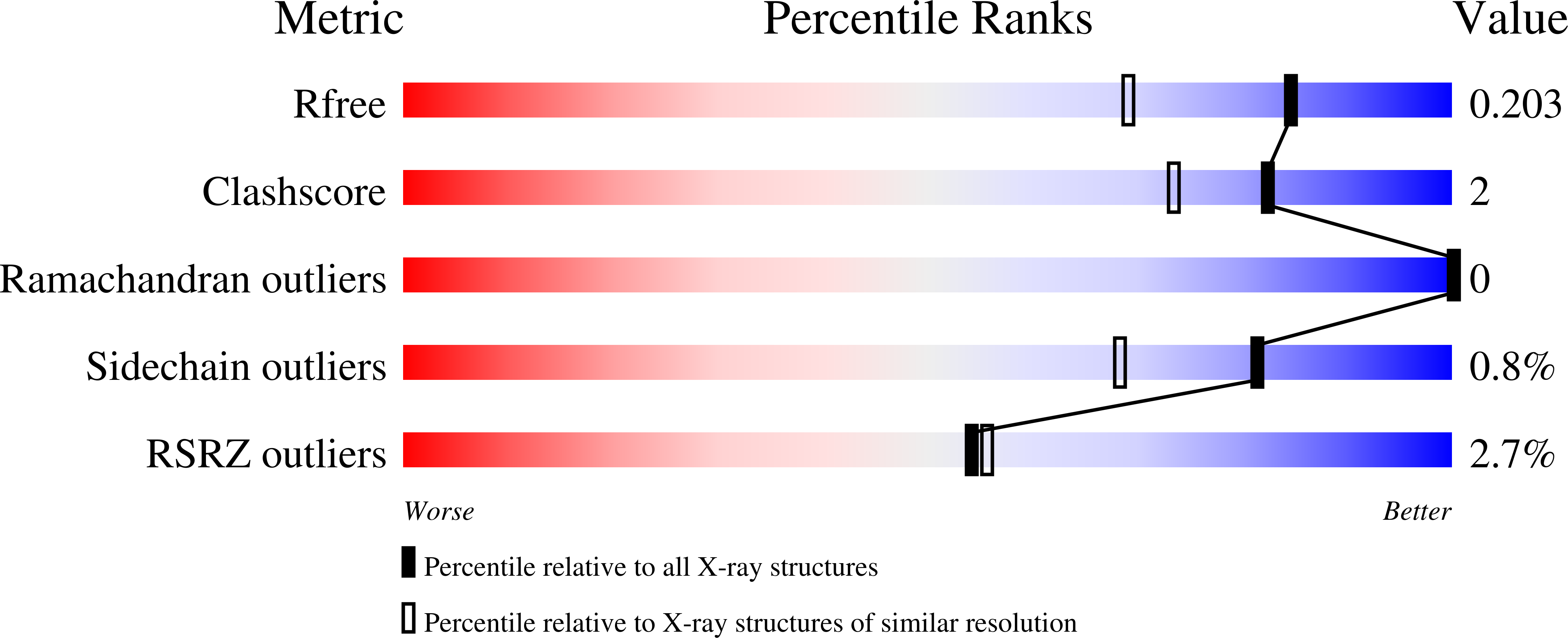
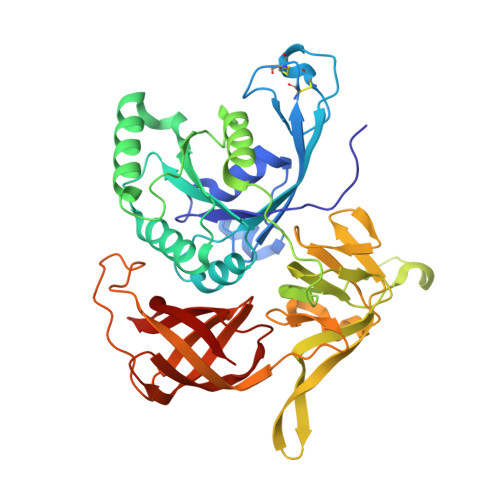
Identification of a second GTP-bound magnesium ion in archaeal initiation factor 2.
Dubiez, E., Aleksandrov, A., Lazennec-Schurdevin, C., Mechulam, Y., Schmitt, E.(2015) Nucleic Acids Res 43: 2946-2957
- PubMed: 25690901
- DOI: https://doi.org/10.1093/nar/gkv053
- Primary Citation of Related Structures:
4RCY, 4RCZ, 4RD0, 4RD1, 4RD2, 4RD3, 4RD4, 4RD6 - PubMed Abstract:
Eukaryotic and archaeal translation initiation processes involve a heterotrimeric GTPase e/aIF2 crucial for accuracy of start codon selection. In eukaryotes, the GTPase activity of eIF2 is assisted by a GTPase-activating protein (GAP), eIF5. In archaea, orthologs of eIF5 are not found and aIF2 GTPase activity is thought to be non-assisted. However, no in vitro GTPase activity of the archaeal factor has been reported to date. Here, we show that aIF2 significantly hydrolyses GTP in vitro. Within aIF2γ, H97, corresponding to the catalytic histidine found in other translational GTPases, and D19, from the GKT loop, both participate in this activity. Several high-resolution crystal structures were determined to get insight into GTP hydrolysis by aIF2γ. In particular, a crystal structure of the H97A mutant was obtained in the presence of non-hydrolyzed GTP. This structure reveals the presence of a second magnesium ion bound to GTP and D19. Quantum chemical/molecular mechanical simulations support the idea that the second magnesium ion may assist GTP hydrolysis by helping to neutralize the developing negative charge in the transition state. These results are discussed in light of the absence of an identified GAP in archaea to assist GTP hydrolysis on aIF2.
Organizational Affiliation:
Laboratoire de Biochimie, Unité Mixte de Recherche 7654, Ecole Polytechnique, Centre National de la Recherche Scientifique, F-91128 Palaiseau cedex, France.