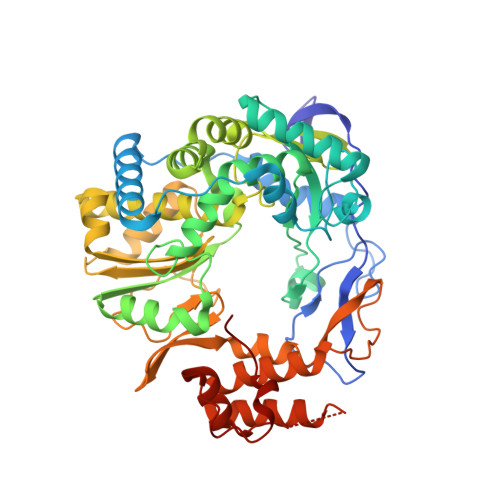
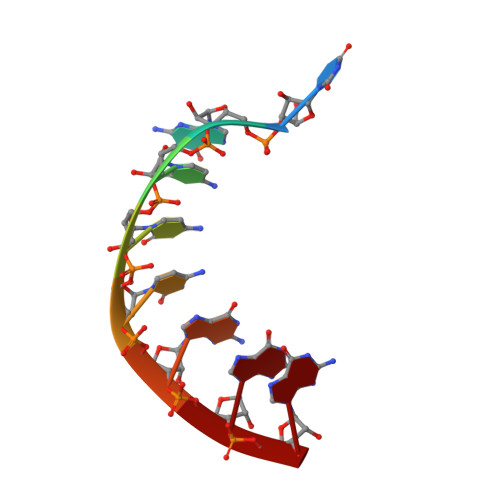
Structure of a backtracked state reveals conformational changes similar to the state following nucleotide incorporation in human norovirus polymerase.
Zamyatkin, D., Rao, C., Hoffarth, E., Jurca, G., Rho, H., Parra, F., Grochulski, P., Ng, K.K.(2014) Acta Crystallogr D Biol Crystallogr 70: 3099-3109
- PubMed: 25478829
- DOI: https://doi.org/10.1107/S1399004714021518
- Primary Citation of Related Structures:
4QPX - PubMed Abstract:
The RNA-dependent RNA polymerase (RdRP) from norovirus (NV) genogroup II has previously been crystallized as an apoenzyme (APO1) in multiple crystal forms, as well as as a pre-incorporation ternary complex (PRE1) bound to Mn(2+), various nucleoside triphosphates and an RNA primer-template duplex in an orthorhombic crystal form. When crystallized under near-identical conditions with a slightly different RNA primer/template duplex, however, the enzyme-RNA complex forms tetragonal crystals (anisotropic data, dmin ≃ 1.9 Å) containing a complex with the primer/template bound in a backtracked state (BACK1) similar to a post-incorporation complex (POST1) in a step of the enzymatic cycle immediately following nucleotidyl transfer. The BACK1 conformation shows that the terminal nucleotide of the primer binds in a manner similar to the nucleoside triphosphate seen in the PRE1 complex, even though the terminal two phosphoryl groups in the triphosphate moiety are absent and a covalent bond is present between the α-phosphoryl group of the terminal nucleotide and the 3'-oxygen of the penultimate nucleotide residue. The two manganese ions bound at the active site coordinate to conserved Asp residues and the bridging phosphoryl group of the terminal nucleotide. Surprisingly, the conformation of the thumb domain in BACK1 resembles the open APO1 state more than the closed conformation seen in PRE1. The BACK1 complex thus reveals a hybrid state in which the active site is closed while the thumb domain is open. Comparison of the APO1, PRE1 and BACK1 structures of NV polymerase helps to reveal a more complete and complex pathway of conformational changes within a single RdRP enzyme system. These conformational changes lend insight into the mechanism of RNA translocation following nucleotidyl transfer and suggest novel approaches for the development of antiviral inhibitors.
Organizational Affiliation:
Department of Biological Sciences, University of Calgary, 2500 University Drive NW, Calgary, Alberta T2N 1N4, Canada.