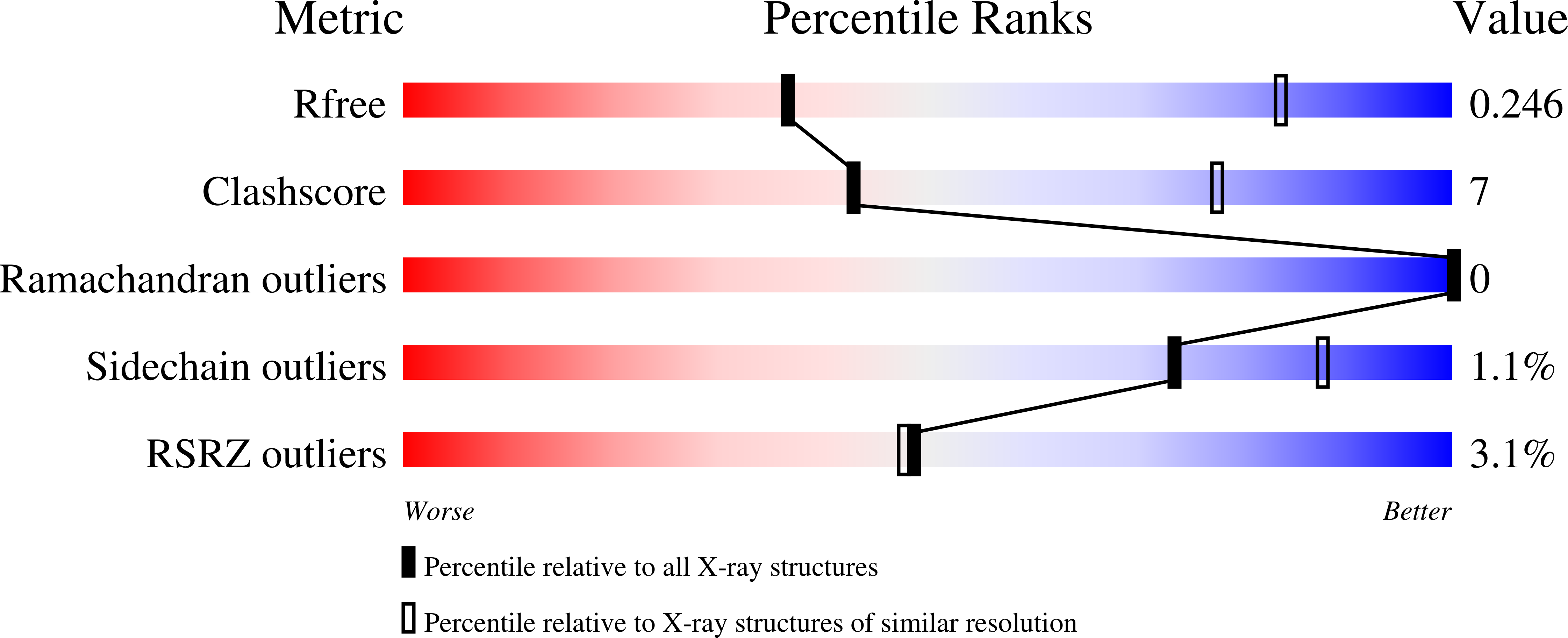
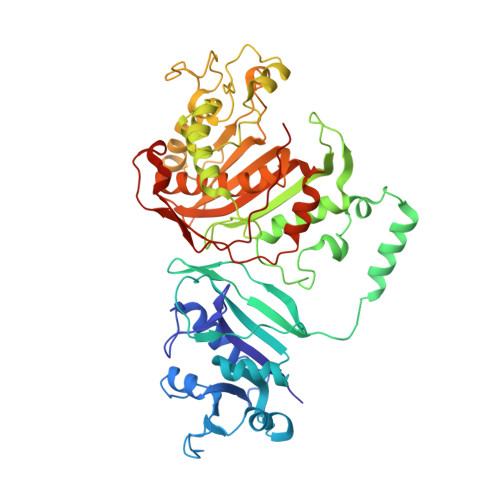
Structural studies provide clues for analog design of specific inhibitors of Cryptosporidium hominis thymidylate synthase-dihydrofolate reductase.
Kumar, V.P., Cisneros, J.A., Frey, K.M., Castellanos-Gonzalez, A., Wang, Y., Gangjee, A., White, A.C., Jorgensen, W.L., Anderson, K.S.(2014) Bioorg Med Chem Lett 24: 4158-4161
- PubMed: 25127103
- DOI: https://doi.org/10.1016/j.bmcl.2014.07.049
- Primary Citation of Related Structures:
4Q0D, 4Q0E - PubMed Abstract:
Cryptosporidium is the causative agent of a gastrointestinal disease, cryptosporidiosis, which is often fatal in immunocompromised individuals and children. Thymidylate synthase (TS) and dihydrofolate reductase (DHFR) are essential enzymes in the folate biosynthesis pathway and are well established as drug targets in cancer, bacterial infections, and malaria. Cryptosporidium hominis has a bifunctional thymidylate synthase and dihydrofolate reductase enzyme, compared to separate enzymes in the host. We evaluated lead compound 1 from a novel series of antifolates, 2-amino-4-oxo-5-substituted pyrrolo[2,3-d]pyrimidines as an inhibitor of Cryptosporidium hominis thymidylate synthase with selectivity over the human enzyme. Complementing the enzyme inhibition compound 1 also has anti-cryptosporidial activity in cell culture. A crystal structure with compound 1 bound to the TS active site is discussed in terms of several van der Waals, hydrophobic and hydrogen bond interactions with the protein residues and the substrate analog 5-fluorodeoxyuridine monophosphate (TS), cofactor NADPH and inhibitor methotrexate (DHFR). Another crystal structure in complex with compound 1 bound in both the TS and DHFR active sites is also reported here. The crystal structures provide clues for analog design and for the design of ChTS-DHFR specific inhibitors.
Organizational Affiliation:
Department of Pharmacology, Yale University School of Medicine, 333 Cedar Street, New Haven, CT 06520, USA.