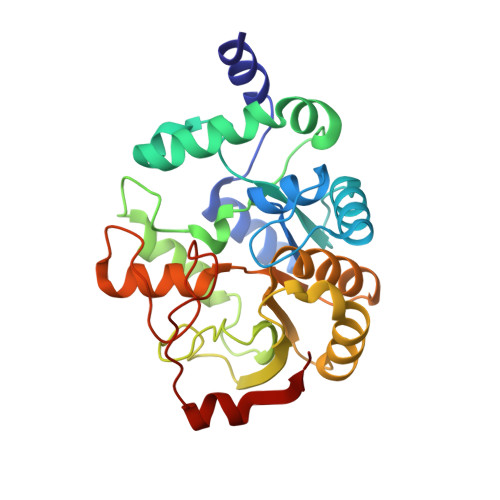
The highly conserved domain of unknown function 1792 has a distinct glycosyltransferase fold.
Zhang, H., Zhu, F., Yang, T., Ding, L., Zhou, M., Li, J., Haslam, S.M., Dell, A., Erlandsen, H., Wu, H.(2014) Nat Commun 5: 4339-4339
- PubMed: 25023666
- DOI: https://doi.org/10.1038/ncomms5339
- Primary Citation of Related Structures:
4PFX, 4PHR, 4PHS - PubMed Abstract:
More than 33,000 glycosyltransferases have been identified. Structural studies, however, have only revealed two distinct glycosyltransferase (GT) folds, GT-A and GT-B. Here we report a 1.34-Å resolution X-ray crystallographic structure of a previously uncharacterized 'domain of unknown function' 1792 (DUF1792) and show that the domain adopts a new fold and is required for glycosylation of a family of serine-rich repeat streptococcal adhesins. Biochemical studies reveal that the domain is a glucosyltransferase, and it catalyses the transfer of glucose to the branch point of the hexasaccharide O-linked to the serine-rich repeat of the bacterial adhesin, Fap1 of Streptococcus parasanguinis. DUF1792 homologues from both Gram-positive and Gram-negative bacteria also exhibit the activity. Thus, DUF1792 represents a new family of glycosyltransferases; therefore, we designate it as a GT-D glycosyltransferase fold. As the domain is highly conserved in bacteria and not found in eukaryotes, it can be explored as a new antibacterial target.
Organizational Affiliation:
Departments of Pediatric Dentistry, Microbiology, Schools of Dentistry and Medicine, University of Alabama at Birmingham, Birmingham, Alabama 35294, USA.