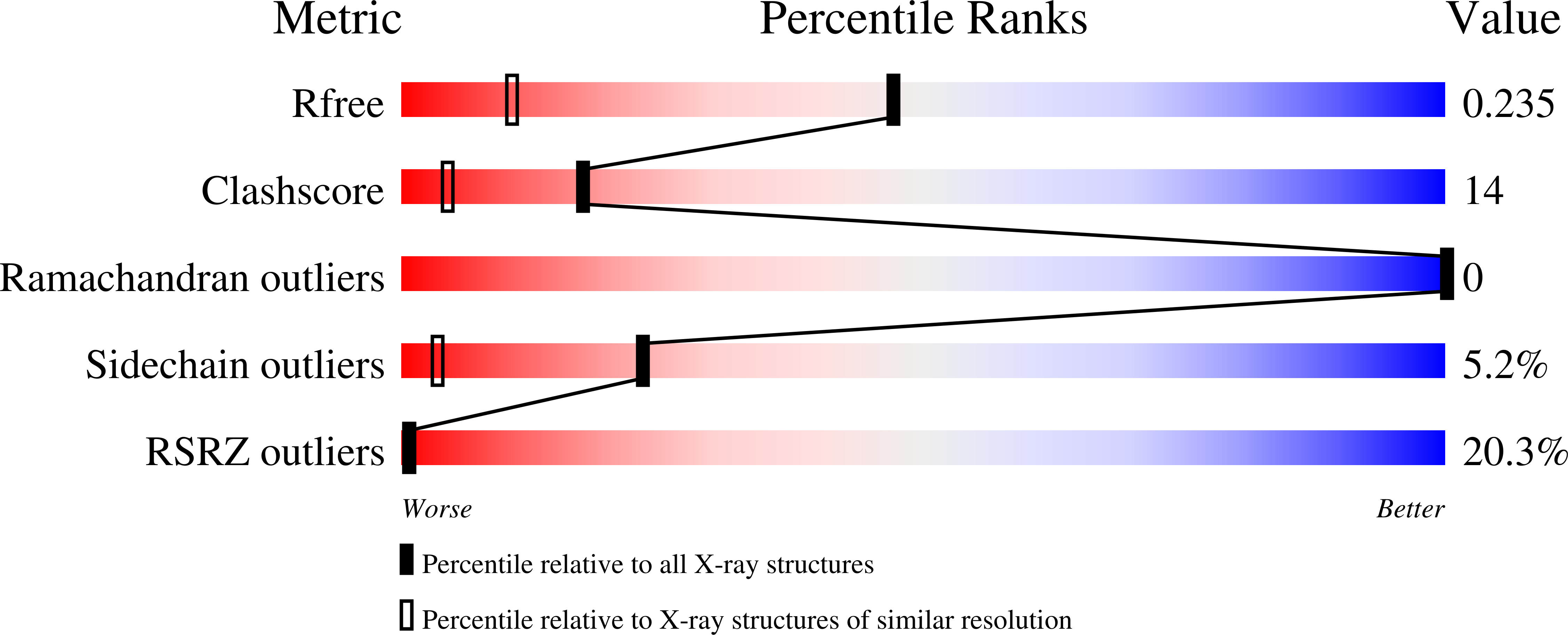
Fluorine Modulates Species Selectivity in the Triazolopyrimidine Class of Plasmodium falciparum Dihydroorotate Dehydrogenase Inhibitors.
Deng, X., Kokkonda, S., El Mazouni, F., White, J., Burrows, J.N., Kaminsky, W., Charman, S.A., Matthews, D., Rathod, P.K., Phillips, M.A.(2014) J Med Chem 57: 5381-5394
- PubMed: 24801997
- DOI: https://doi.org/10.1021/jm500481t
- Primary Citation of Related Structures:
4OQV, 4ORI, 4ORM - PubMed Abstract:
Malaria is one of the most serious global infectious diseases. The pyrimidine biosynthetic enzyme Plasmodium falciparum dihydroorotate dehydrogenase (PfDHODH) is an important target for antimalarial chemotherapy. We describe a detailed analysis of protein-ligand interactions between DHODH and a triazolopyrimidine-based inhibitor series to explore the effects of fluorine on affinity and species selectivity. We show that increasing fluorination dramatically increases binding to mammalian DHODHs, leading to a loss of species selectivity. Triazolopyrimidines bind Plasmodium and mammalian DHODHs in overlapping but distinct binding sites. Key hydrogen-bond and stacking interactions underlying strong binding to PfDHODH are absent in the mammalian enzymes. Increasing fluorine substitution leads to an increase in the entropic contribution to binding, suggesting that strong binding to mammalian DHODH is a consequence of an enhanced hydrophobic effect upon binding to an apolar pocket. We conclude that hydrophobic interactions between fluorine and hydrocarbons provide significant binding energy to protein-ligand interactions. Our studies define the requirements for species-selective binding to PfDHODH and show that the triazolopyrimidine scaffold can alternatively be tuned to inhibit human DHODH, an important target for autoimmune diseases.
Organizational Affiliation:
Department of Pharmacology, University of Texas Southwestern Medical Center at Dallas , 6001 Forest Park Boulevard, Dallas, Texas 75390-9041, United States.