Kinetic and structural characterization of an alternatively spliced variant of human mitochondrial 5'(3')-deoxyribonucleotidase.
Pachl, P., Fabry, M., Veverka, V., Brynda, J., Rezacova, P.(2015) J Enzyme Inhib Med Chem 30: 63-68
- PubMed: 24506201
- DOI: https://doi.org/10.3109/14756366.2013.879577
- Primary Citation of Related Structures:
4MUM - PubMed Abstract:
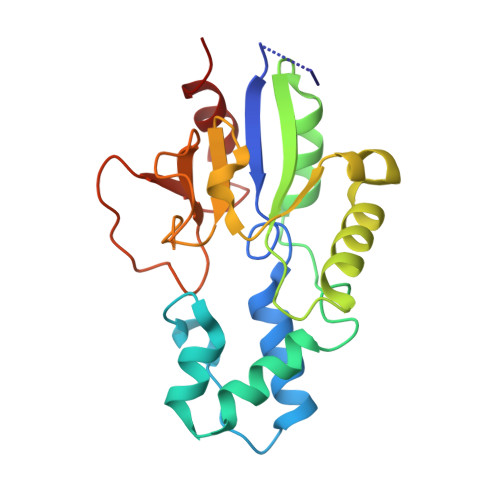
Human mitochondrial 5'(3')-deoxyribonucleotidase (mdN) catalyzes dephosphorylation of nucleoside monophosphates, and thus helps maintain homeostasis of deoxynucleosides required for mitochondrial DNA synthesis. Mature mdN is a 23-kDa dimeric protein with highest expression levels in the heart, brain and skeletal muscle. We have identified an alternative splice variant of the mdN gene containing an 18-nucleotide insertion encoding 6 amino acids (GKWPAT) at the 3'-end of the penultimate exon 4. We recombinantly expressed this enzyme variant and characterized its biochemical and kinetic properties as well as its three-dimensional structure. Our high-resolution (1.27 Å) crystal structure revealed that the insertion forms a loop located in the vicinity of the active site pocket and affects enzyme kinetic parameters as well as protein thermal stability.
Organizational Affiliation:
Institute of Organic Chemistry and Biochemistry and.