Active Form of the Protein Kinase CK2 alpha 2 beta 2 Holoenzyme Is a Strong Complex with Symmetric Architecture.
Lolli, G., Ranchio, A., Battistutta, R.(2014) ACS Chem Biol 9: 366-371
- PubMed: 24175891
- DOI: https://doi.org/10.1021/cb400771y
- Primary Citation of Related Structures:
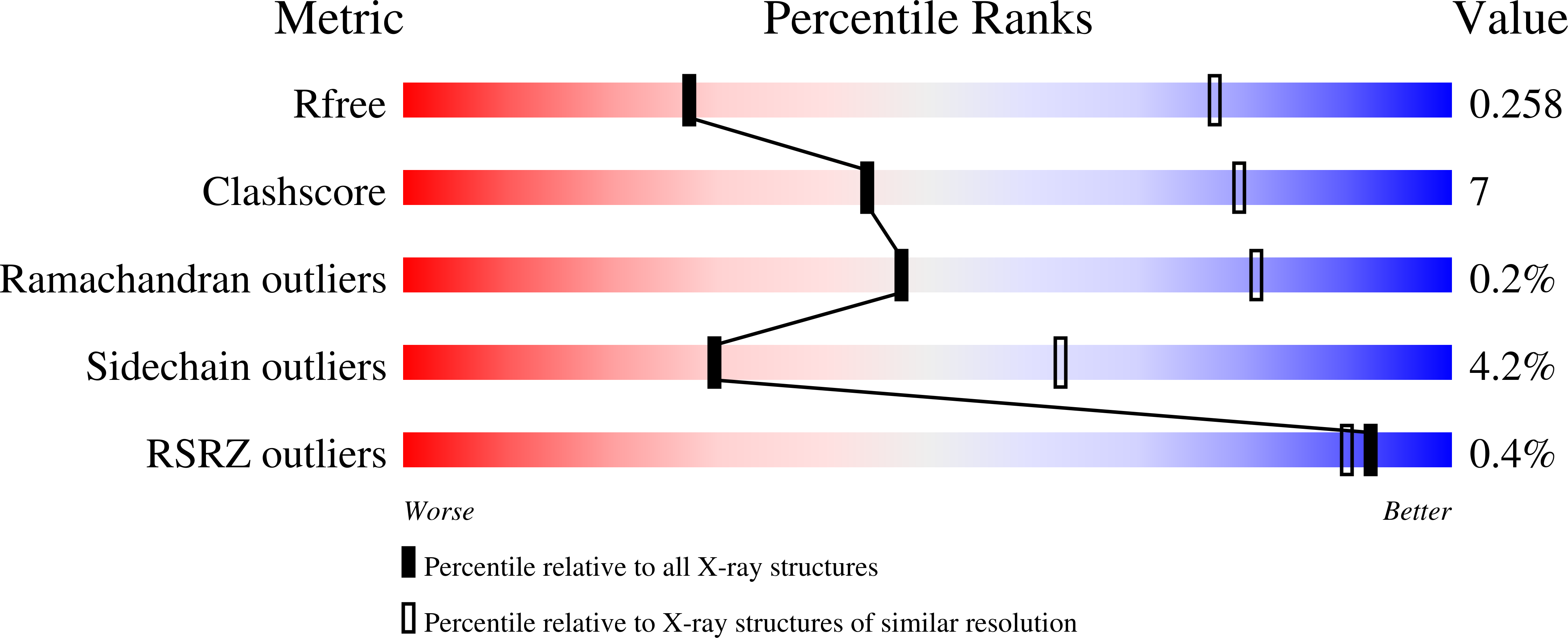
4MD7, 4MD8, 4MD9 - PubMed Abstract:
CK2 is a protein kinase essential for cell viability whose activity is altered in several cancers. Its mechanisms of regulation differ from those common to other eukaryotic protein kinases and are not entirely established yet. Here we present crystal structures of the monomeric form of the α2β2 holoenzyme that allow refining a formerly proposed structural model for activity regulation by oligomerization. Previous crystal structures of the CK2 holoenzyme show an asymmetric arrangement of the two α catalytic subunits around the obligate β2 regulatory subunits. Asymmetric α2β2 tetramers are organized in trimeric rings that correspond to inactive forms of the enzyme. The new crystal structures presented here reveal the symmetric architecture of the isolated active tetramers. The dimension and the nature of the α/β interfaces configure the holoenzyme as a strong complex that does not spontaneously dissociate in solution, in accordance with the low dissociation constant (∼4 nM).
Organizational Affiliation:
Department of Chemical Sciences, University of Padua , via Marzolo 1, 35131 Padova, Italy.