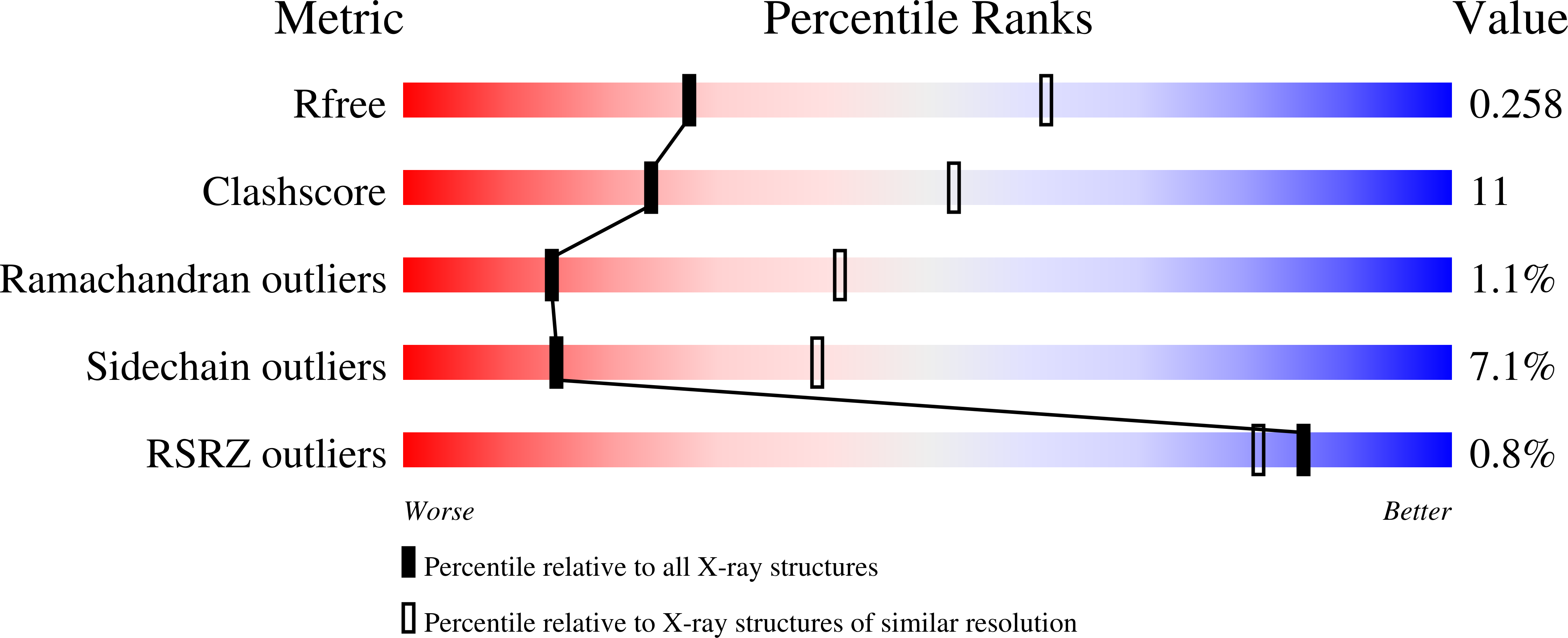
Structure of 2-keto-3-deoxy-D-manno-octulosonate-8-phosphate synthase from Pseudomonas aeruginosa.
Nelson, S.K., Kelleher, A., Robinson, G., Reiling, S., Asojo, O.A.(2013) Acta Crystallogr Sect F Struct Biol Cryst Commun 69: 1084-1088
- PubMed: 24100553
- DOI: https://doi.org/10.1107/S1744309113023993
- Primary Citation of Related Structures:
4LU0 - PubMed Abstract:
Pseudomonas aeruginosa is a major cause of opportunistic infection and is resistant to most antibiotics. As part of efforts to generate much-needed new antibiotics, structural studies of enzymes that are critical for the virulence of P. aeruginosa but are absent in mammals have been initiated. 2-Keto-3-deoxy-D-manno-octulosonate-8-phosphate synthase (KDO8Ps), also known as 2-dehydro-3-deoxyphosphooctonate aldolase, is vital for the survival and virulence of P. aeruginosa. This enzyme catalyzes a key step in the synthesis of the lipopolysaccharide (LPS) of most Gram-negative bacteria: the condensation reaction between phosphoenolpyruvate (PEP) and arabinose 5-phosphate to produce 2-keto-3-deoxy-D-manno-octulosonate-8-phosphate (KDO8P). This step is vital for the proper synthesis and assembly of LPS and the survival of P. aeruginosa. Here, the recombinant expression, purification and crystal structure of KDO8Ps from P. aeruginosa are presented. Orthorhombic crystals were obtained by vapor diffusion in sitting drops in the presence of 1 mM phosphoenlpyruvate. The structure reveals the prototypical α/β TIM-barrel structure expected from this family of enzymes and contains a tetramer in the asymmetric unit.
Organizational Affiliation:
National School of Tropical Medicine, Baylor College of Medicine, 1102 Bates Avenue, Houston, TX 77030, USA.