Structural Insights into the Functions of TBK1 in Innate Antimicrobial Immunity.
Shu, C., Sankaran, B., Chaton, C.T., Herr, A.B., Mishra, A., Peng, J., Li, P.(2013) Structure 21: 1137-1148
- PubMed: 23746807
- DOI: https://doi.org/10.1016/j.str.2013.04.025
- Primary Citation of Related Structures:
4JL9, 4JLC - PubMed Abstract:
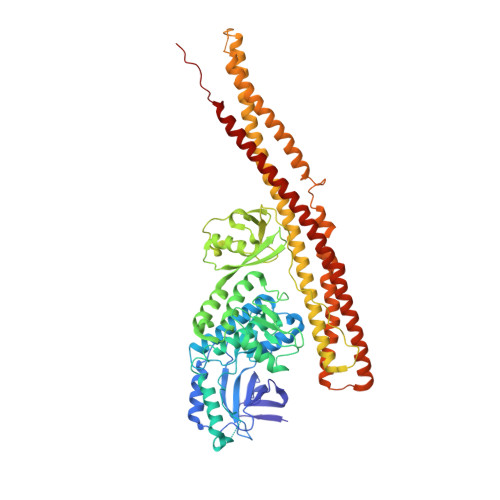
Tank-binding kinase 1 (TBK1) is a serine/threonine protein-kinase mediating innate antimicrobial immunity. TBK1 is involved in the signaling of TLRs, RLRs, and STING-mediated sensing of cytosolic DNA. Stimulation of these receptors results in the activation of TBK1, which phosphorylates interferon regulatory factor (IRF)-3. Phosphorylated IRF-3 translocates into the nucleus to initiate the transcription of the interferon (IFN)-β gene. Here, we show that TBK1 is activated by autophosphorylation at residue Ser172. Structures of TBK1 bound to two inhibitors showed that TBK1 has the IκB kinase fold with three distinct domains: the kinase domain, the ubiquitin-like domain, and the scaffold and dimerization domain. However, the overall structures of the TBK1 monomer and its dimer are different from IKKβ in the arrangements of the three domains and in dimer formation. Phosphorylation of IRF-3 by TBK1 in vitro results in its oligomerization, and phosphorylation of residue Ser386 plays a key role in IRF-3 activation.
Organizational Affiliation:
Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX 77843-2128, USA.