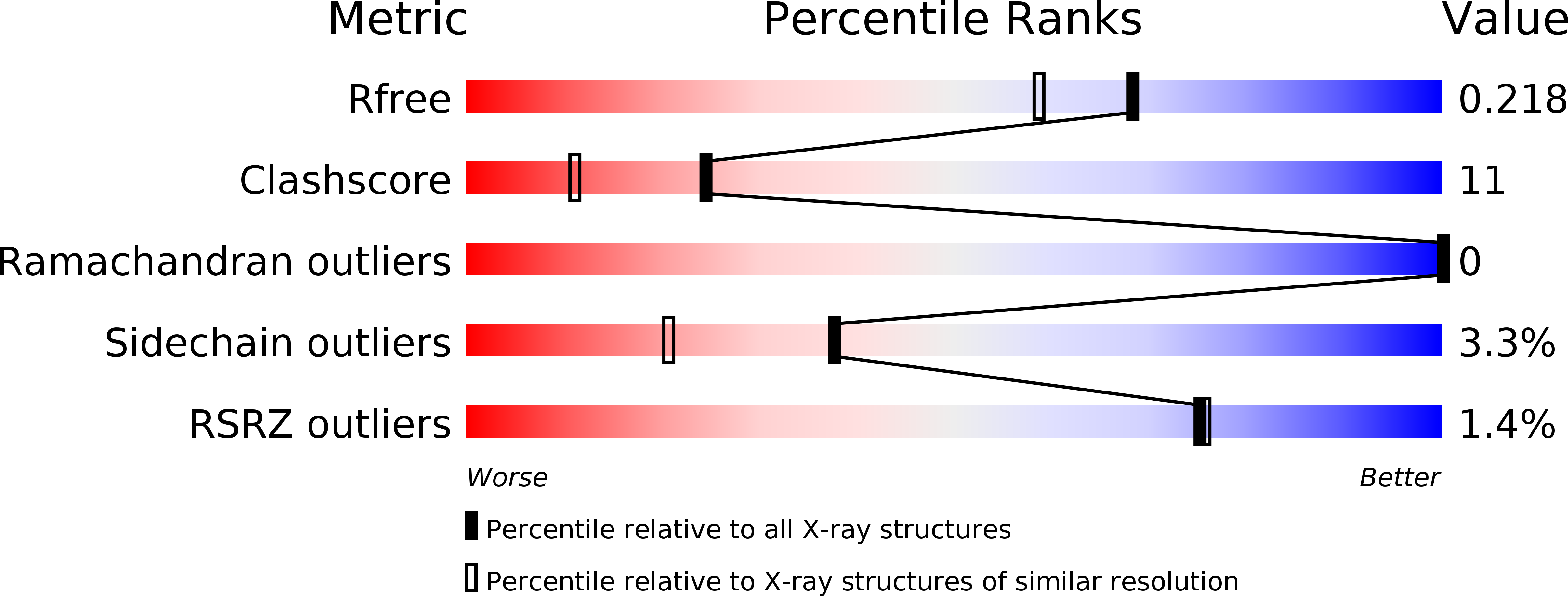
Crystallographic Observation of pH-Induced Conformational Changes in the Amyelois transitella Pheromone-Binding Protein AtraPBP1.
di Luccio, E., Ishida, Y., Leal, W.S., Wilson, D.K.(2013) PLoS One 8: e53840-e53840
- PubMed: 23418423
- DOI: https://doi.org/10.1371/journal.pone.0053840
- Primary Citation of Related Structures:
4INW, 4INX - PubMed Abstract:
The navel orangeworm, Amyelois transitella is a major agricultural pest causing large losses in a variety of tree crops. Control of this insect pest may be achieved by interfering with olfactory pathways to block detection of female-produced sex pheromones and consequently, disrupt mating. The first component of this pathway is the pheromone-binding protein AtraPBP1, which recognizes the pheromone and presents it to the odorant receptor housed in a sensory neuron of the male antennae. Release of the ligand depends on a pH-induced conformational change associated with the acidity of the membrane surface. To characterize this conformational change and to understand how pheromones bind, we have determined the high resolution crystal structures of AtraPBP1 in complex with two main constituents of the sex pheromone, i.e., (11Z,13Z)-hexadecadienal and (11Z,13Z)-hexadecadienol. Comparison with the structure of the unliganded form demonstrates a large ∼90° movement of the C-terminal helix which is observed in other pheromone- or odorant-binding proteins accompanied by an unpredicted 37° displacement of the N-terminal helix. Molecular dynamic trajectories suggest that the conformational change of the α1 helix facilitates the movement of the C-terminal helix.
Organizational Affiliation:
Department of Molecular and Cellular Biology, University of California Davis, Davis, California, United States of America.