4-Substituted-2,3,5,6-tetrafluorobenzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, XII, and XIII.
Dudutiene, V., Zubriene, A., Smirnov, A., Gylyte, J., Timm, D., Manakova, E., Grazulis, S., Matulis, D.(2013) Bioorg Med Chem 21: 2093-2106
- PubMed: 23394791
- DOI: https://doi.org/10.1016/j.bmc.2013.01.008
- Primary Citation of Related Structures:
4HT0, 4HT2, 4HU1 - PubMed Abstract:
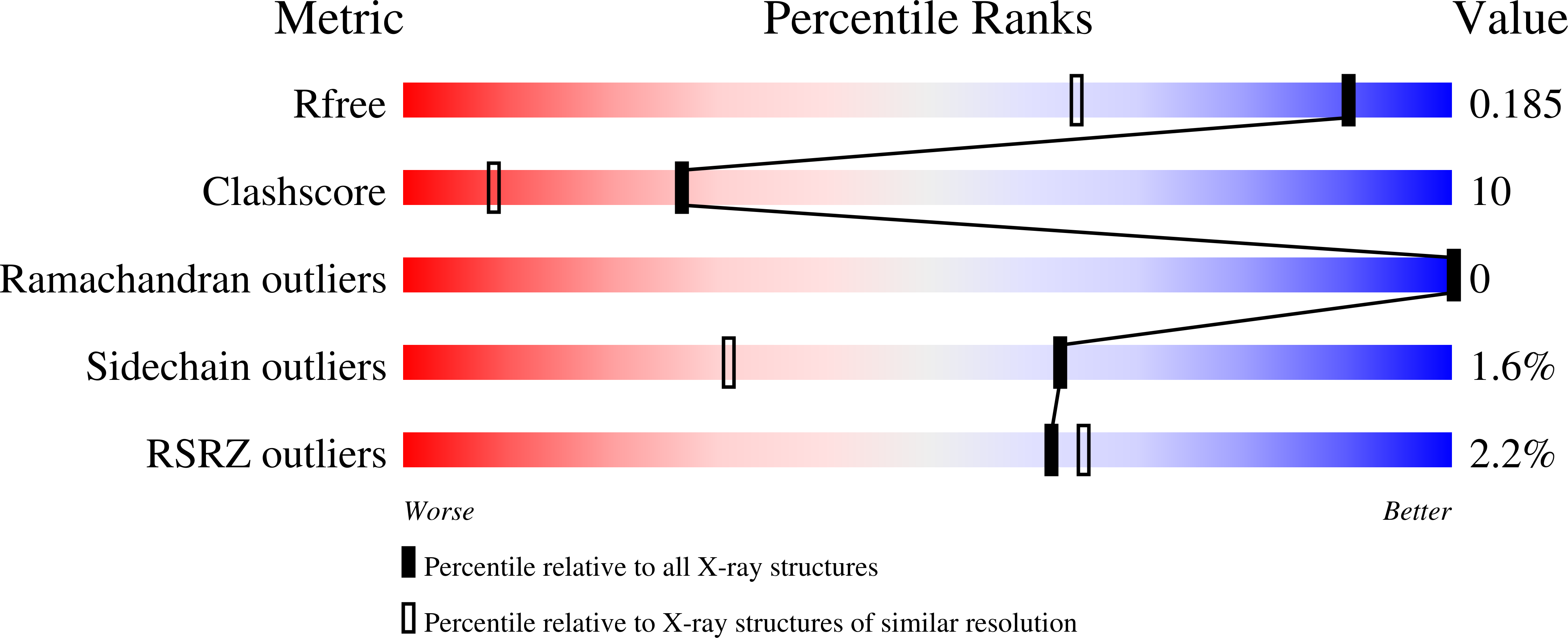
A series of 4-substituted-2,3,5,6-tetrafluorobenezenesulfonamides were synthesized and their binding potencies as inhibitors of recombinant human carbonic anhydrase isozymes I, II, VII, XII, and XIII were determined by the thermal shift assay, isothermal titration calorimetry, and stop-flow CO2 hydration assay. All fluorinated benzenesulfonamides exhibited nanomolar binding potency toward tested CAs and fluorinated benzenesulfonamides posessed higher binding potency than non-fluorinated compounds. The crystal structures of 4-[(4,6-dimethylpyrimidin-2-yl)thio]-2,3,5,6-tetrafluorobenzenesulfonamide in complex with CA II and CA XII, and 2,3,5,6-tetrafluoro-4-[(2-hydroxyethyl)sulfonyl]benzenesulfonamide in complex with CA XIII were determined. The observed dissociation constants for several fluorinated compounds reached subnanomolar range for CA I isozyme. The affinity and the selectivity of the compounds towards tested isozymes are presented.
Organizational Affiliation:
Department of Biothermodynamics and Drug Design,Vilnius University Institute of Biotechnology, Graičiūno 8, Vilnius LT-02241, Lithuania.