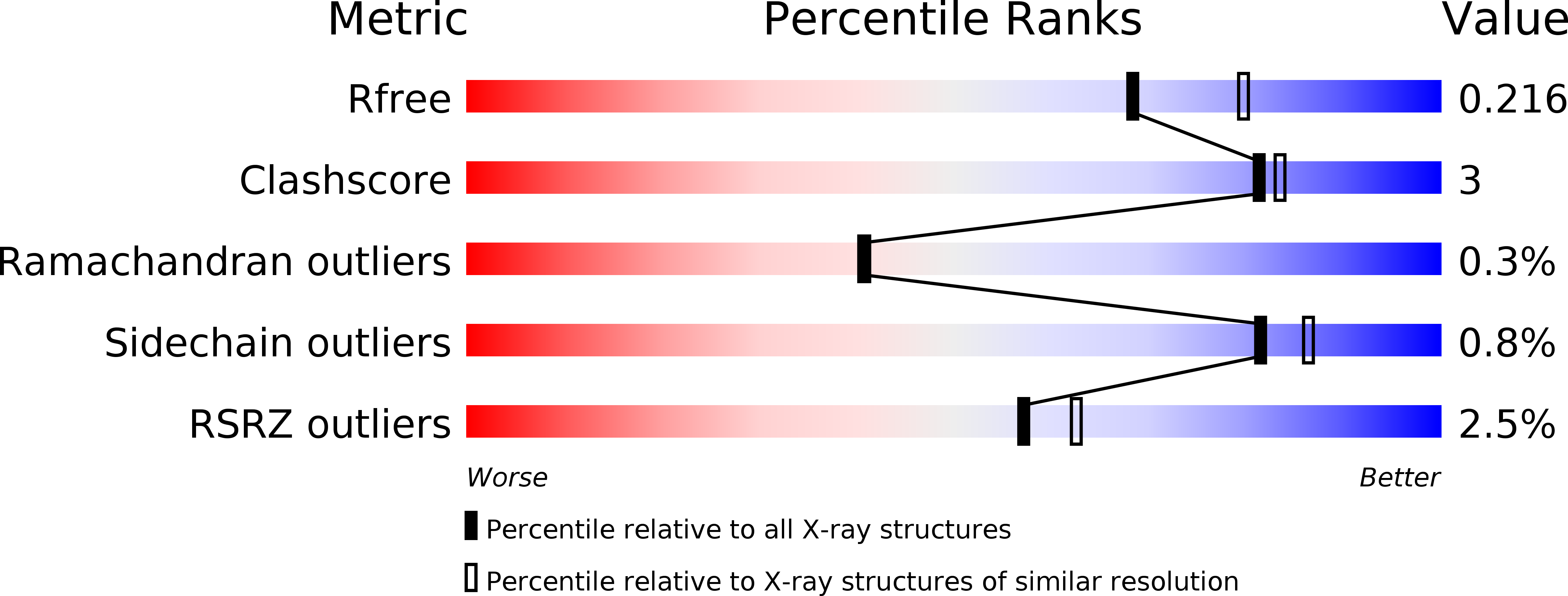
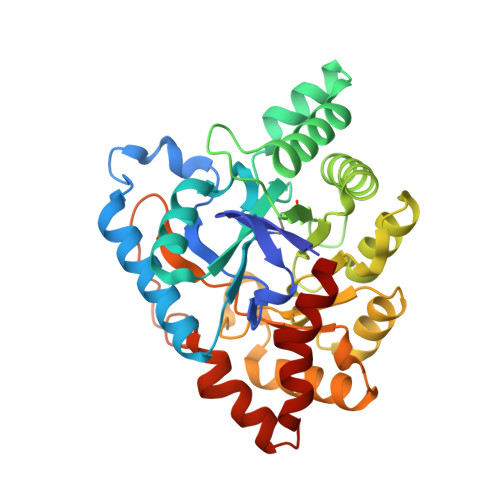
Structural evidence of a productive active site architecture for an evolved quorum-quenching GKL lactonase.
Xue, B., Chow, J.Y., Baldansuren, A., Yap, L.L., Gan, Y.H., Dikanov, S.A., Robinson, R.C., Yew, W.S.(2013) Biochemistry 52: 2359-2370
- PubMed: 23461395
- DOI: https://doi.org/10.1021/bi4000904
- Primary Citation of Related Structures:
4H9T, 4H9U, 4H9V, 4H9X, 4H9Y, 4H9Z, 4HA0 - PubMed Abstract:
The in vitro evolution and engineering of quorum-quenching lactonases with enhanced reactivities was achieved using a thermostable GKL enzyme as a template, yielding the E101G/R230C GKL mutant with increased catalytic activity and a broadened substrate range [Chow, J. Y., Xue, B., Lee, K. H., Tung, A., Wu, L., Robinson, R. C., and Yew, W. S. (2010) J. Biol. Chem. 285, 40911-40920]. This enzyme possesses the (β/α)8-barrel fold and is a member of the PLL (phosphotriesterase-like lactonase) group of enzymes within the amidohydrolase superfamily that hydrolyze N-acyl-homoserine lactones, which mediate the quorum-sensing pathways of bacteria. The structure of the evolved N-butyryl-l-homoserine lactone (substrate)-bound E101G/R230C GKL enzyme was determined, in the presence of the inactivating D266N mutation, to a resolution of 2.2 Å to provide an explanation for the observed rate enhancements. In addition, the substrate-bound structure of the catalytically inactive E101N/D266N mutant of the manganese-reconstituted enzyme was determined to a resolution of 2.1 Å and the structure of the ligand-free, manganese-reconstituted E101N mutant to a resolution of 2.6 Å, and the structures of ligand-free zinc-reconstituted wild-type, E101N, R230D, and E101G/R230C mutants of GKL were determined to resolutions of 2.1, 2.1, 1.9, and 2.0 Å, respectively. In particular, the structure of the evolved E101G/R230C mutant of GKL provides evidence of a catalytically productive active site architecture that contributes to the observed enhancement of catalysis. At high concentrations, wild-type and mutant GKL enzymes are differentially colored, with absorbance maxima in the range of 512-553 nm. The structures of the wild-type and mutant GKL provide a tractable link between the origins of the coloration and the charge-transfer complex between the α-cation and Tyr99 within the enzyme active site. Taken together, this study provides evidence of the modulability of enzymatic catalysis through subtle changes in enzyme active site architecture.
Organizational Affiliation:
Institute of Molecular and Cell Biology, 61 Biopolis Drive, Singapore 138673.