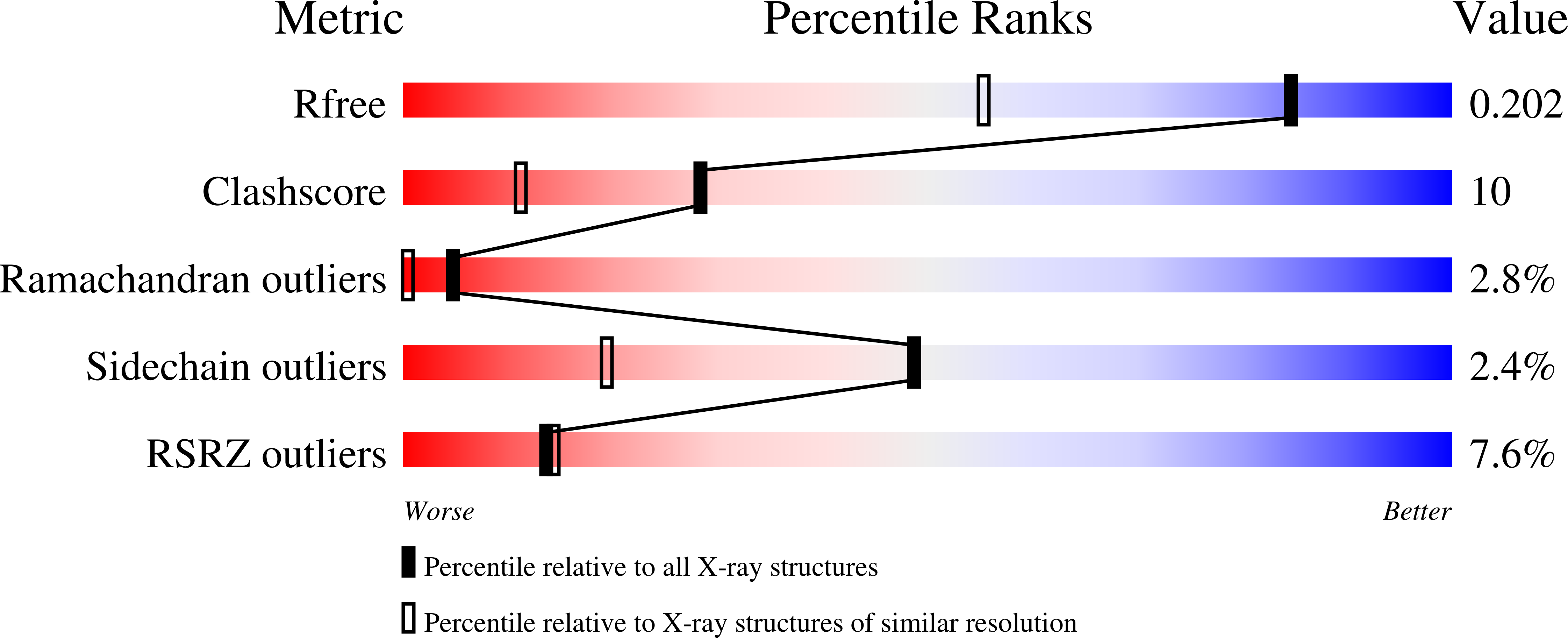
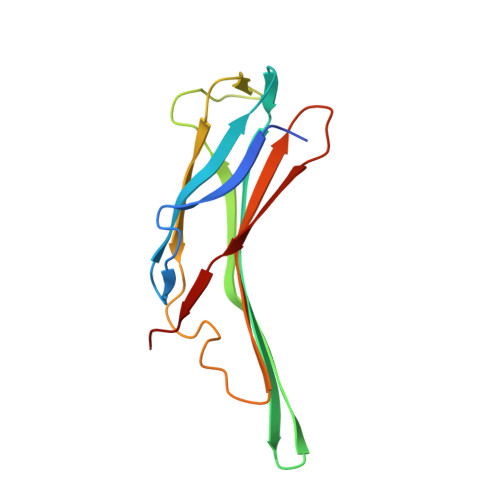
Structure of the N-terminal domain of human thioredoxin-interacting protein.
Polekhina, G., Ascher, D.B., Kok, S.F., Beckham, S., Wilce, M., Waltham, M.(2013) Acta Crystallogr D Biol Crystallogr 69: 333-344
- PubMed: 23519408
- DOI: https://doi.org/10.1107/S0907444912047099
- Primary Citation of Related Structures:
4GEI, 4GEJ - PubMed Abstract:
Thioredoxin-interacting protein (TXNIP) is one of the six known α-arrestins and has recently received considerable attention owing to its involvement in redox signalling and metabolism. Various stress stimuli such as high glucose, heat shock, UV, H2O2 and mechanical stress among others robustly induce the expression of TXNIP, resulting in the sequestration and inactivation of thioredoxin, which in turn leads to cellular oxidative stress. While TXNIP is the only α-arrestin known to bind thioredoxin, TXNIP and two other α-arrestins, Arrdc4 and Arrdc3, have been implicated in metabolism. Furthermore, owing to its roles in the pathologies of diabetes and cardiovascular disease, TXNIP is considered to be a promising drug target. Based on their amino-acid sequences, TXNIP and the other α-arrestins are remotely related to β-arrestins. Here, the crystal structure of the N-terminal domain of TXNIP is reported. It provides the first structural information on any of the α-arrestins and reveals that although TXNIP adopts a β-arrestin fold as predicted, it is structurally more similar to Vps26 proteins than to β-arrestins, while sharing below 15% pairwise sequence identity with either.
Organizational Affiliation:
Centre for Cancer Research, Monash Institute of Medical Research, Monash University, 27-31 Wright Street, Clayton, VIC 3168, Australia. galina.polekhina@monash.edu