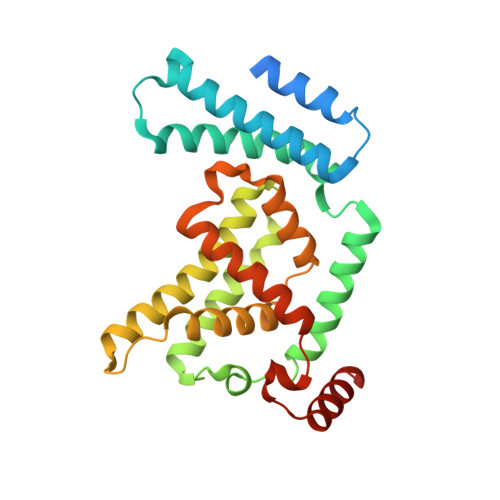
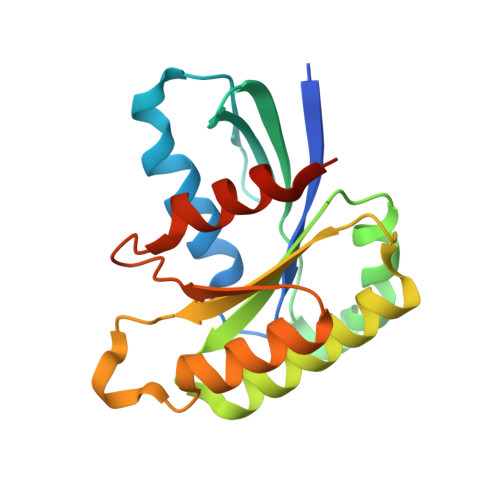
Direct metal recognition by guanine nucleotide-exchange factor in the initial step of the exchange reaction
Uejima, T., Ihara, K., Sunada, M., Kawasaki, M., Ueda, T., Kato, R., Nakano, A., Wakatsuki, S.(2013) Acta Crystallogr D Biol Crystallogr 69: 345-351
- PubMed: 23519409
- DOI: https://doi.org/10.1107/S0907444912047294
- Primary Citation of Related Structures:
4G01 - PubMed Abstract:
Rab small GTPases regulate vesicle transport in eukaryotes by interacting with various effectors. Guanine nucleotide-exchange factor (GEF) catalyzes the transition from inactive GDP-bound Rab to active GTP-bound Rab. The existence of several GDP-bound intermediates containing the Arabidopsis thaliana Rab5 homologue ARA7 and the GEF VPS9a prior to the formation of a nucleotide-free binary complex has been proposed [Uejima et al. (2010), J. Biol. Chem. 285, 36689-36697]. During this process, VPS9a directly interacts with the β-phosphate of GDP and the P-loop lysine of ARA7 via a catalytically important aspartate finger, which promotes the release of GDP from ARA7. However, it is unclear how VPS9a removes Mg2+ from ARA7 before forming the GDP-bound ternary complex. Here, the structure of the ARA7-GDP-Ca2+-VPS9a complex is reported, in which the aspartate finger directly coordinates the divalent metal ion. Ca2+ is bound to the canonical Mg2+-binding site, coordinated by the β-phosphate of GDP and the P-loop serine of ARA7. Unexpectedly, Ca2+ is further coordinated by the aspartate finger and the main chain of VPS9a. This structure may represent the earliest intermediate step in the GEF-catalyzed nucleotide-exchange reaction of ARA7 before the metal-free GDP-bound intermediates are created.
Organizational Affiliation:
Structural Biology Research Center, Institute of Materials Structure Science, High Energy Accelerator Research Organization, 1-1 Oho, Tsukuba, Ibaraki 305-0801, Japan. tamami.uejima@kek.jp