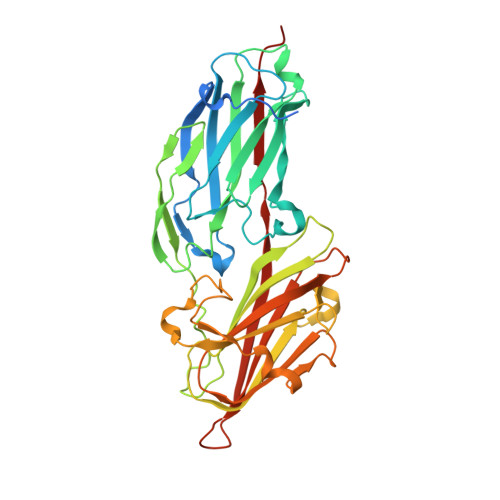
Crystal Structures Reveal the Multi-Ligand Binding Mechanism of Staphylococcus aureus ClfB
Xiang, H., Feng, Y., Wang, J.W., Liu, B., Chen, Y.G., Liu, L., Deng, X.M., Yang, M.J.(2012) PLoS Pathog 8: e1002751-e1002751
- PubMed: 22719251
- DOI: https://doi.org/10.1371/journal.ppat.1002751
- Primary Citation of Related Structures:
4F1Z, 4F20, 4F24, 4F27 - PubMed Abstract:
Staphylococcus aureus (S. aureus) pathogenesis is a complex process involving a diverse array of extracellular and cell wall components. ClfB, an MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules) family surface protein, described as a fibrinogen-binding clumping factor, is a key determinant of S. aureus nasal colonization, but the molecular basis for ClfB-ligand recognition remains unknown. In this study, we solved the crystal structures of apo-ClfB and its complexes with fibrinogen α (Fg α) and cytokeratin 10 (CK10) peptides. Structural comparison revealed a conserved glycine-serine-rich (GSR) ClfB binding motif (GSSGXGXXG) within the ligands, which was also found in other human proteins such as Engrailed protein, TCF20 and Dermokine proteins. Interaction between Dermokine and ClfB was confirmed by subsequent binding assays. The crystal structure of ClfB complexed with a 15-residue peptide derived from Dermokine revealed the same peptide binding mode of ClfB as identified in the crystal structures of ClfB-Fg α and ClfB-CK10. The results presented here highlight the multi-ligand binding property of ClfB, which is very distinct from other characterized MSCRAMMs to-date. The adherence of multiple peptides carrying the GSR motif into the same pocket in ClfB is reminiscent of MHC molecules. Our results provide a template for the identification of other molecules targeted by S. aureus during its colonization and infection. We propose that other MSCRAMMs like ClfA and SdrG also possess multi-ligand binding properties.
Organizational Affiliation:
Key Laboratory for Protein Sciences of Ministry of Education, Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China.