An Unusual Role for a Mobile Flavin in StaC-like Indolocarbazole Biosynthetic Enzymes.
Goldman, P.J., Ryan, K.S., Hamill, M.J., Howard-Jones, A.R., Walsh, C.T., Elliott, S.J., Drennan, C.L.(2012) Chem Biol 19: 855-865
- PubMed: 22840773
- DOI: https://doi.org/10.1016/j.chembiol.2012.05.016
- Primary Citation of Related Structures:
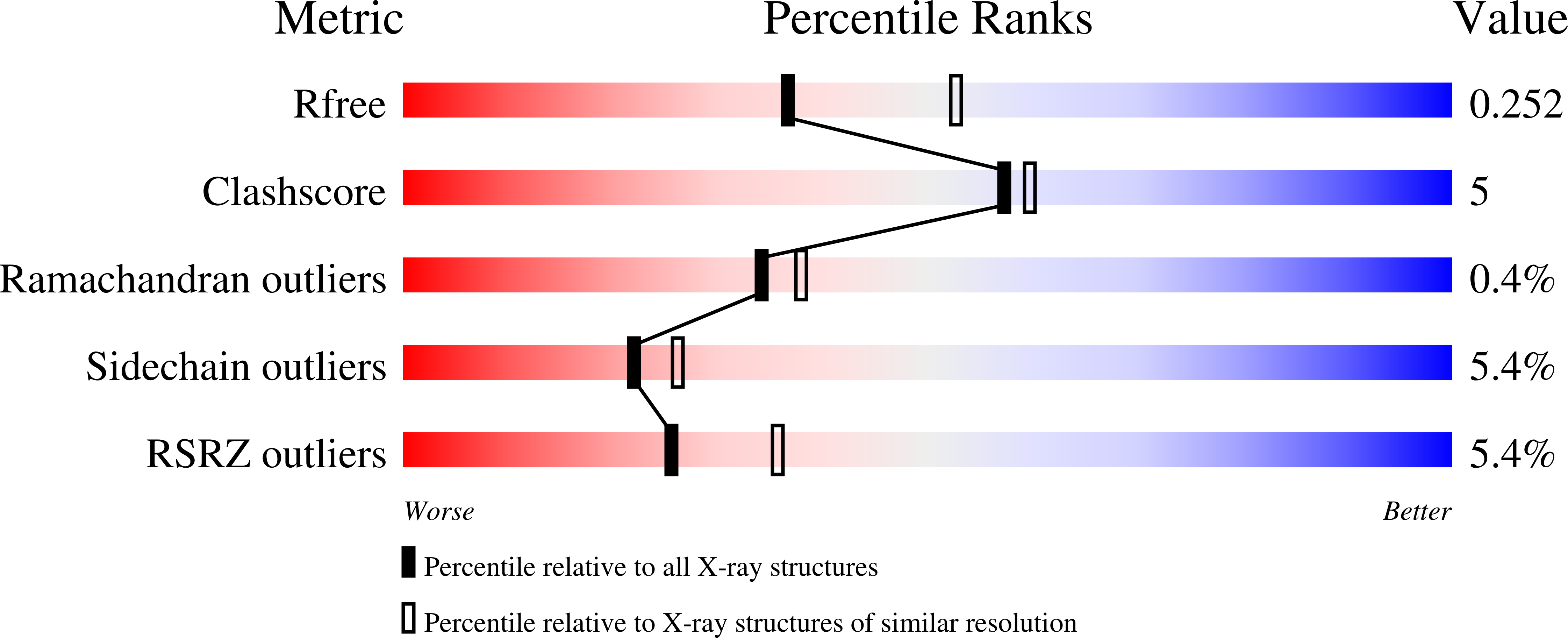
4EIP, 4EIQ - PubMed Abstract:
The indolocarbazole biosynthetic enzymes StaC, InkE, RebC, and AtmC mediate the degree of oxidation of chromopyrrolic acid on route to the natural products staurosporine, K252a, rebeccamycin, and AT2433-A1, respectively. Here, we show that StaC and InkE, which mediate a net 4-electron oxidation, bind FAD with a micromolar K(d), whereas RebC and AtmC, which mediate a net 8-electron oxidation, bind FAD with a nanomolar K(d) while displaying the same FAD redox properties. We further create RebC-10x, a RebC protein with ten StaC-like amino acid substitutions outside of previously characterized FAD-binding motifs and the complementary StaC-10x. We find that these mutations mediate both FAD affinity and product specificity, with RebC-10x displaying higher StaC activity than StaC itself. X-ray structures of this StaC catalyst identify the substrate of StaC as 7-carboxy-K252c and suggest a unique mechanism for this FAD-dependent enzyme.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139, USA.