Tertiary Structure-Function Analysis Reveals the Pathogenic Signaling Potentiation Mechanism of Helicobacter pylori Oncogenic Effector CagA
Hayashi, T., Senda, M., Morohashi, H., Higashi, H., Horio, M., Kashiba, Y., Nagase, L., Sasaya, D., Shimizu, T., Venugopalan, N., Kumeta, H., Noda, N.N., Inagaki, F., Senda, T., Hatakeyama, M.(2012) Cell Host Microbe 12: 20-33
- PubMed: 22817985
- DOI: https://doi.org/10.1016/j.chom.2012.05.010
- Primary Citation of Related Structures:
4DVY, 4DVZ - PubMed Abstract:
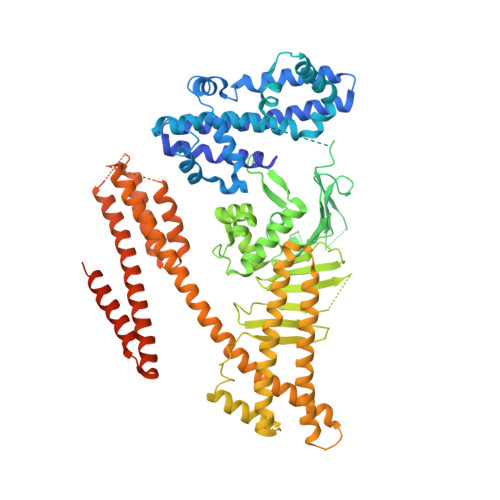
The Helicobacter pylori type IV secretion effector CagA is a major bacterial virulence determinant and critical for gastric carcinogenesis. Upon delivery into gastric epithelial cells, CagA localizes to the inner face of the plasma membrane, where it acts as a pathogenic scaffold/hub that promiscuously recruits host proteins to potentiate oncogenic signaling. We find that CagA comprises a structured N-terminal region and an intrinsically disordered C-terminal region that directs versatile protein interactions. X-ray crystallographic analysis of the N-terminal CagA fragment (residues 1-876) revealed that the region has a structure comprised of three discrete domains. Domain I constitutes a mobile CagA N terminus, while Domain II tethers CagA to the plasma membrane by interacting with membrane phosphatidylserine. Domain III interacts intramolecularly with the intrinsically disordered C-terminal region, and this interaction potentiates the pathogenic scaffold/hub function of CagA. The present work provides a tertiary-structural basis for the pathophysiological/oncogenic action of H. pylori CagA.
Organizational Affiliation:
Division of Microbiology, Graduate School of Medicine, University of Tokyo, Japan.