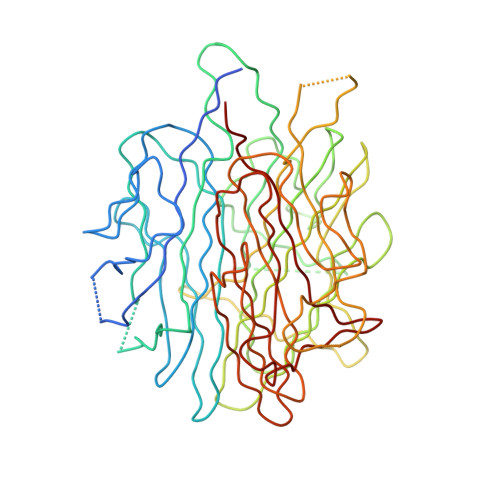
Crystal structure of a single-chain trimer of human adiponectin globular domain.
Min, X., Lemon, B., Tang, J., Liu, Q., Zhang, R., Walker, N., Li, Y., Wang, Z.(2012) FEBS Lett 586: 912-917
- PubMed: 22449980
- DOI: https://doi.org/10.1016/j.febslet.2012.02.024
- Primary Citation of Related Structures:
4DOU - PubMed Abstract:
Adiponectin is increasingly recognized as a potential therapeutic agent for the treatment of diabetes and other metabolic diseases. It circulates in plasma as homotrimers and higher-order oliogomers of homotrimers. To facilitate the production of active recombinant adiponectin as a therapeutic tool, we designed a single-chain globular domain adiponectin (sc-gAd) in which three monomer sequences are linked together in tandem to form one contiguous polypeptide. Here, we present the crystal structure of human sc-gAd at 2.0Å resolution. The structure reveals a similar trimeric topology to that of mouse gAd protein. Trimer formation is further rigidified by three calcium ions.
Organizational Affiliation:
Department of Molecular Structure, Amgen Inc., South San Francisco, CA 94080, USA.