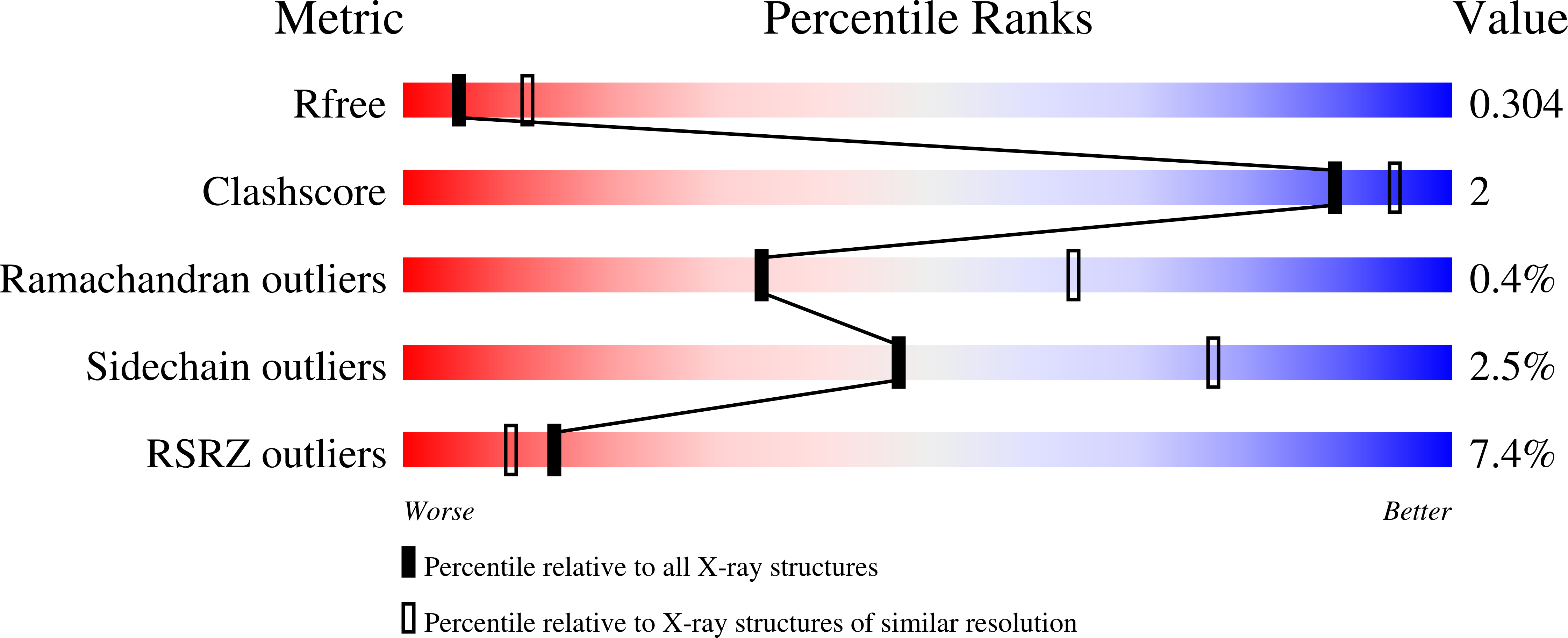
The Crystal Structure of Erwinia Amylovora Levansucrase Provides a Snapshot of the Products of Sucrose Hydrolysis Trapped Into the Active Site.
Wuerges, J., Caputi, L., Cianci, M., Boivin, S., Meijers, R., Benini, S.(2015) J Struct Biol 191: 290
- PubMed: 26208466
- DOI: https://doi.org/10.1016/j.jsb.2015.07.010
- Primary Citation of Related Structures:
4D47 - PubMed Abstract:
Levansucrases are members of the glycoside hydrolase family and catalyse both the hydrolysis of the substrate sucrose and the transfer of fructosyl units to acceptor molecules. In the presence of sufficient sucrose, this may either lead to the production of fructooligosaccharides or fructose polymers. Aim of this study is to rationalise the differences in the polymerisation properties of bacterial levansucrases and in particular to identify structural features that determine different product spectrum in the levansucrase of the Gram-negative bacterium Erwinia amylovora (Ea Lsc, EC 2.4.1.10) as compared to Gram-positive bacteria such as Bacillus subtilis levansucrase. Ea is an enterobacterial pathogen responsible for the Fire Blight disease in rosaceous plants (e.g., apple and pear) with considerable interest for the agricultural industry. The crystal structure of Ea Lsc was solved at 2.77 Å resolution and compared to those of other fructosyltransferases from Gram-positive and Gram-negative bacteria. We propose the structural features, determining the different reaction products, to reside in just a few loops at the rim of the active site funnel. Moreover we propose that loop 8 may have a role in product length determination in Gluconacetobacter diazotrophicus LsdA and Microbacterium saccharophilum FFase. The Ea Lsc structure shows for the first time the products of sucrose hydrolysis still bound in the active site.
Organizational Affiliation:
Bioorganic Chemistry and Bio-Crystallography laboratory (B(2)Cl), Faculty of Science and Technology, Free University of Bolzano, 39100 Bolzano, Italy. Electronic address: jochen.wuerges@unibz.it.