Cryo-Electron Microscopy of Tubular Arrays of HIV-1 Gag Resolves Structures Essential for Immature Virus Assembly.
Bharat, T.A.M., Castillo Menendez, L.R., Hagen, W.J.H., Lux, V., Igonet, S., Schorb, M., Schur, F.K.M., Krausslich, H., Briggs, J.A.G.(2014) Proc Natl Acad Sci U S A 111: 8233
- PubMed: 24843179
- DOI: https://doi.org/10.1073/pnas.1401455111
- Primary Citation of Related Structures:
4COC, 4COP, 4D1K - PubMed Abstract:
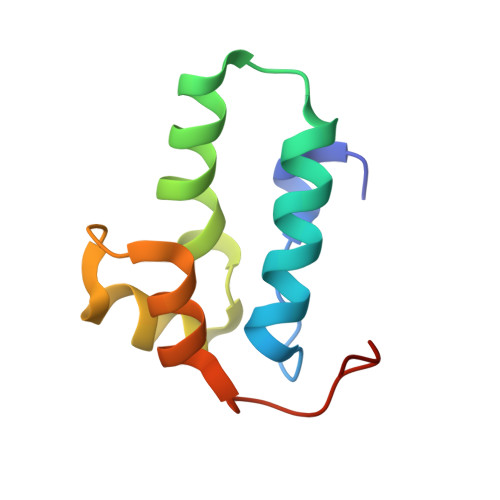
The assembly of HIV-1 is mediated by oligomerization of the major structural polyprotein, Gag, into a hexameric protein lattice at the plasma membrane of the infected cell. This leads to budding and release of progeny immature virus particles. Subsequent proteolytic cleavage of Gag triggers rearrangement of the particles to form mature infectious virions. Obtaining a structural model of the assembled lattice of Gag within immature virus particles is necessary to understand the interactions that mediate assembly of HIV-1 particles in the infected cell, and to describe the substrate that is subsequently cleaved by the viral protease. An 8-Å resolution structure of an immature virus-like tubular array assembled from a Gag-derived protein of the related retrovirus Mason-Pfizer monkey virus (M-PMV) has previously been reported, and a model for the arrangement of the HIV-1 capsid (CA) domains has been generated based on homology to this structure. Here we have assembled tubular arrays of a HIV-1 Gag-derived protein with an immature-like arrangement of the C-terminal CA domains and have solved their structure by using hybrid cryo-EM and tomography analysis. The structure reveals the arrangement of the C-terminal domain of CA within an immature-like HIV-1 Gag lattice, and provides, to our knowledge, the first high-resolution view of the region immediately downstream of CA, which is essential for assembly, and is significantly different from the respective region in M-PMV. Our results reveal a hollow column of density for this region in HIV-1 that is compatible with the presence of a six-helix bundle at this position.
Organizational Affiliation:
Structural and Computational Biology Unit, European Molecular Biology Laboratory, 69117 Heidelberg, Germany;Molecular Medicine Partnership Unit, European Molecular Biology Laboratory/Universitätsklinikum Heidelberg, Heidelberg, Germany;Structural Studies Division, MRC Laboratory of Molecular Biology, Cambridge CB2 0QH, United Kingdom;