6-Substituted Pyrrolo[2,3-d]pyrimidine Thienoyl Regioisomers as Targeted Antifolates for Folate Receptor alpha and the Proton-Coupled Folate Transporter in Human Tumors.
Wang, L., Wallace, A., Raghavan, S., Deis, S.M., Wilson, M.R., Yang, S., Polin, L., White, K., Kushner, J., Orr, S., George, C., O'Connor, C., Hou, Z., Mitchell-Ryan, S., Dann, C.E., Matherly, L.H., Gangjee, A.(2015) J Med Chem 58: 6938-6959
- PubMed: 26317331
- DOI: https://doi.org/10.1021/acs.jmedchem.5b00801
- Primary Citation of Related Structures:
4ZZ1, 4ZZ2, 4ZZ3 - PubMed Abstract:
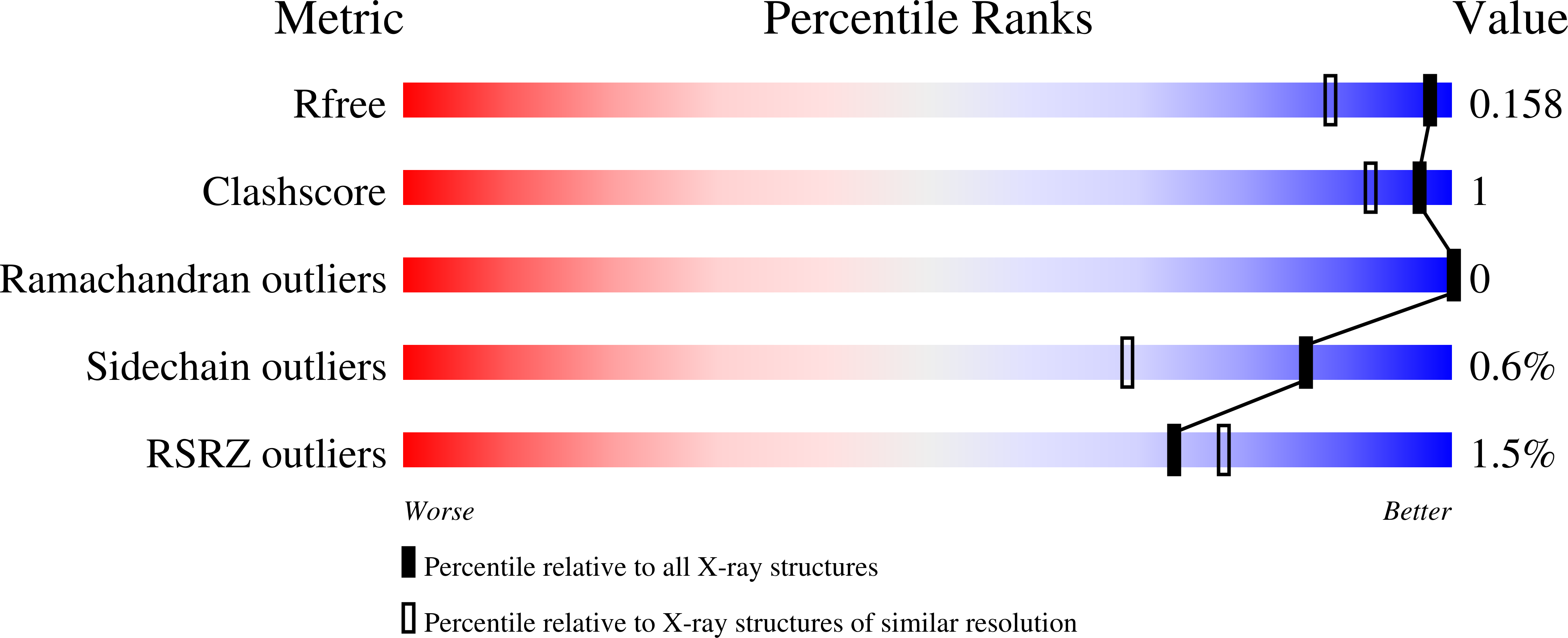
2-Amino-4-oxo-6-substituted-pyrrolo[2,3-d]pyrimidine antifolate thiophene regioisomers of AGF94 (4) with a thienoyl side chain and three-carbon bridge lengths [AGF150 (5) and AGF154 (7)] were synthesized as potential antitumor agents. These analogues inhibited proliferation of Chinese hamster ovary (CHO) sublines expressing folate receptors (FRs) α or β (IC50s < 1 nM) or the proton-coupled folate transporter (PCFT) (IC50 < 7 nM). Compounds 5 and 7 inhibited KB, IGROV1, and SKOV3 human tumor cells at subnanomolar concentrations, reflecting both FRα and PCFT uptake. AGF152 (6) and AGF163 (8), 2,4-diamino-5-substituted-furo[2,3-d]pyrimidine thiophene regioisomers, also inhibited growth of FR-expressing CHO and KB cells. All four analogues inhibited glycinamide ribonucleotide formyltransferase (GARFTase). Crystal structures of human GARFTase complexed with 5 and 7 were reported. In severe combined immunodeficient mice bearing SKOV3 tumors, 7 was efficacious. The selectivity of these compounds for PCFT and for FRα and β over the ubiquitously expressed reduced folate carrier is a paradigm for selective tumor targeting.
Organizational Affiliation:
Division of Medicinal Chemistry, Graduate School of Pharmaceutical Sciences, Duquesne University , 600 Forbes Avenue, Pittsburgh, Pennsylvania 15282, United States.