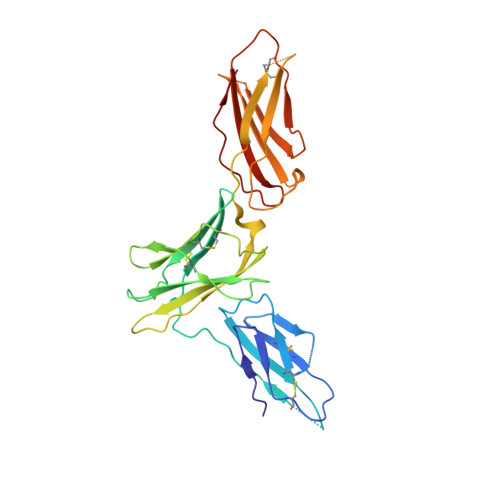
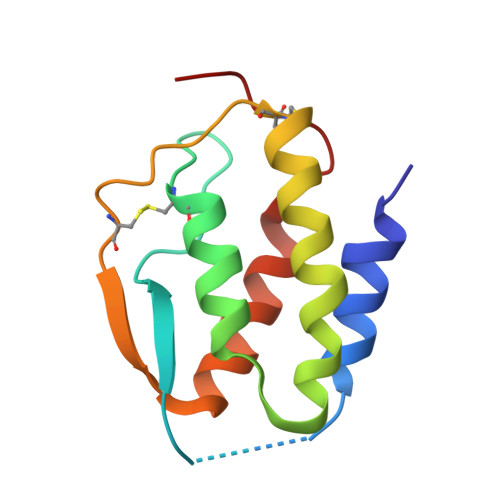
Conformational Changes in the GM-CSF Receptor Suggest a Molecular Mechanism for Affinity Conversion and Receptor Signaling.
Broughton, S.E., Hercus, T.R., Nero, T.L., Dottore, M., McClure, B.J., Dhagat, U., Taing, H., Gorman, M.A., King-Scott, J., Lopez, A.F., Parker, M.W.(2016) Structure 24: 1271-1281
- PubMed: 27396825
- DOI: https://doi.org/10.1016/j.str.2016.05.017
- Primary Citation of Related Structures:
4NKQ, 4RS1 - PubMed Abstract:
The GM-CSF, IL-3, and IL-5 receptors constitute the βc family, playing important roles in inflammation, autoimmunity, and cancer. Typical of heterodimeric type I cytokine receptors, signaling requires recruitment of the shared subunit to the initial cytokine:α subunit binary complex through an affinity conversion mechanism. This critical process is poorly understood due to the paucity of crystal structures of both binary and ternary receptor complexes for the same cytokine. We have now solved the structure of the binary GM-CSF:GMRα complex at 2.8-Å resolution and compared it with the structure of the ternary complex, revealing distinct conformational changes. Guided by these differences we performed mutational and functional studies that, importantly, show GMRα interactions playing a major role in receptor signaling while βc interactions control high-affinity binding. These results support the notion that conformational changes underlie the mechanism of GM-CSF receptor activation and also suggest how related type I cytokine receptors signal.
Organizational Affiliation:
ACRF Rational Drug Discovery Centre, St. Vincent's Institute of Medical Research, Fitzroy, VIC 3065, Australia.