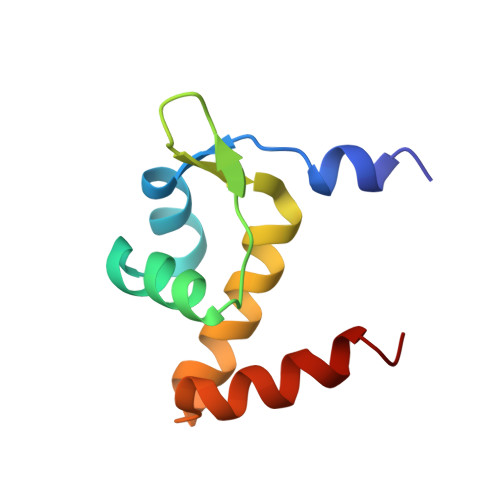
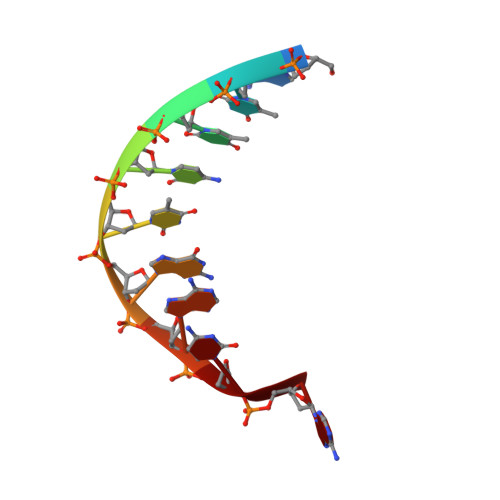
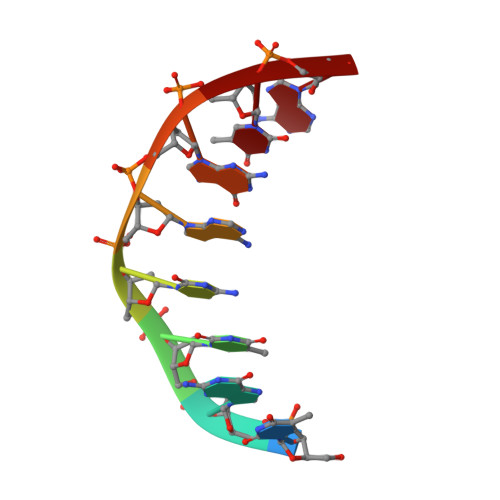
Structures of regulatory machinery reveal novel molecular mechanisms controlling B. subtilis nitrogen homeostasis.
Schumacher, M.A., Chinnam, N.B., Cuthbert, B., Tonthat, N.K., Whitfill, T.(2015) Genes Dev 29: 451-464
- PubMed: 25691471
- DOI: https://doi.org/10.1101/gad.254714.114
- Primary Citation of Related Structures:
4R22, 4R24, 4R25, 4R4E, 4RX6, 4S0R - PubMed Abstract:
All cells must sense and adapt to changing nutrient availability. However, detailed molecular mechanisms coordinating such regulatory pathways remain poorly understood. In Bacillus subtilis, nitrogen homeostasis is controlled by a unique circuitry composed of the regulator TnrA, which is deactivated by feedback-inhibited glutamine synthetase (GS) during nitrogen excess and stabilized by GlnK upon nitrogen depletion, and the repressor GlnR. Here we describe a complete molecular dissection of this network. TnrA and GlnR, the global nitrogen homeostatic transcription regulators, are revealed as founders of a new structural family of dimeric DNA-binding proteins with C-terminal, flexible, effector-binding sensors that modulate their dimerization. Remarkably, the TnrA sensor domains insert into GS intersubunit catalytic pores, destabilizing the TnrA dimer and causing an unprecedented GS dodecamer-to-tetradecamer conversion, which concomitantly deactivates GS. In contrast, each subunit of the GlnK trimer "templates" active TnrA dimers. Unlike TnrA, GlnR sensors mediate an autoinhibitory dimer-destabilizing interaction alleviated by GS, which acts as a GlnR chaperone. Thus, these studies unveil heretofore unseen mechanisms by which inducible sensor domains drive metabolic reprograming in the model Gram-positive bacterium B. subtilis.
Organizational Affiliation:
Department of Biochemistry, Duke University Medical Center, Durham, North Carolina 27710, USA maria.schumacher@duke.edu.