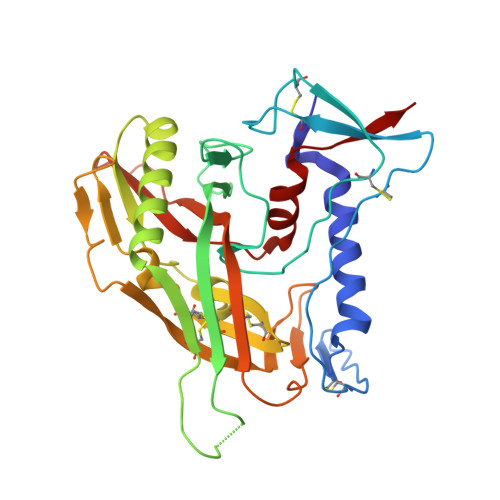
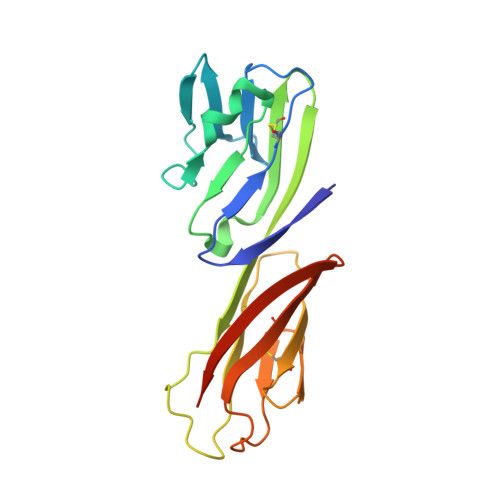
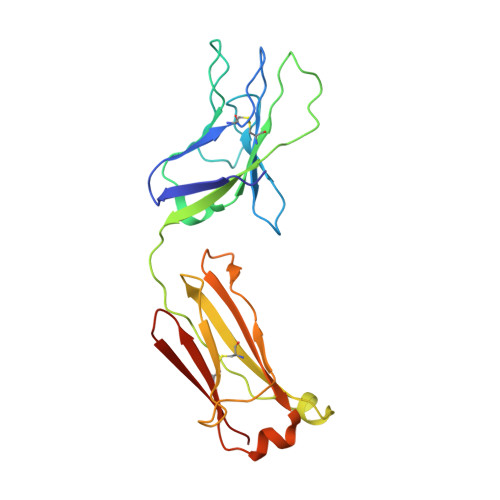
Structural Evolution of Glycan Recognition by a Family of Potent HIV Antibodies.
Garces, F., Sok, D., Kong, L., McBride, R., Kim, H.J., Saye-Francisco, K.F., Julien, J.P., Hua, Y., Cupo, A., Moore, J.P., Paulson, J.C., Ward, A.B., Burton, D.R., Wilson, I.A.(2014) Cell 159: 69-79
- PubMed: 25259921
- DOI: https://doi.org/10.1016/j.cell.2014.09.009
- Primary Citation of Related Structures:
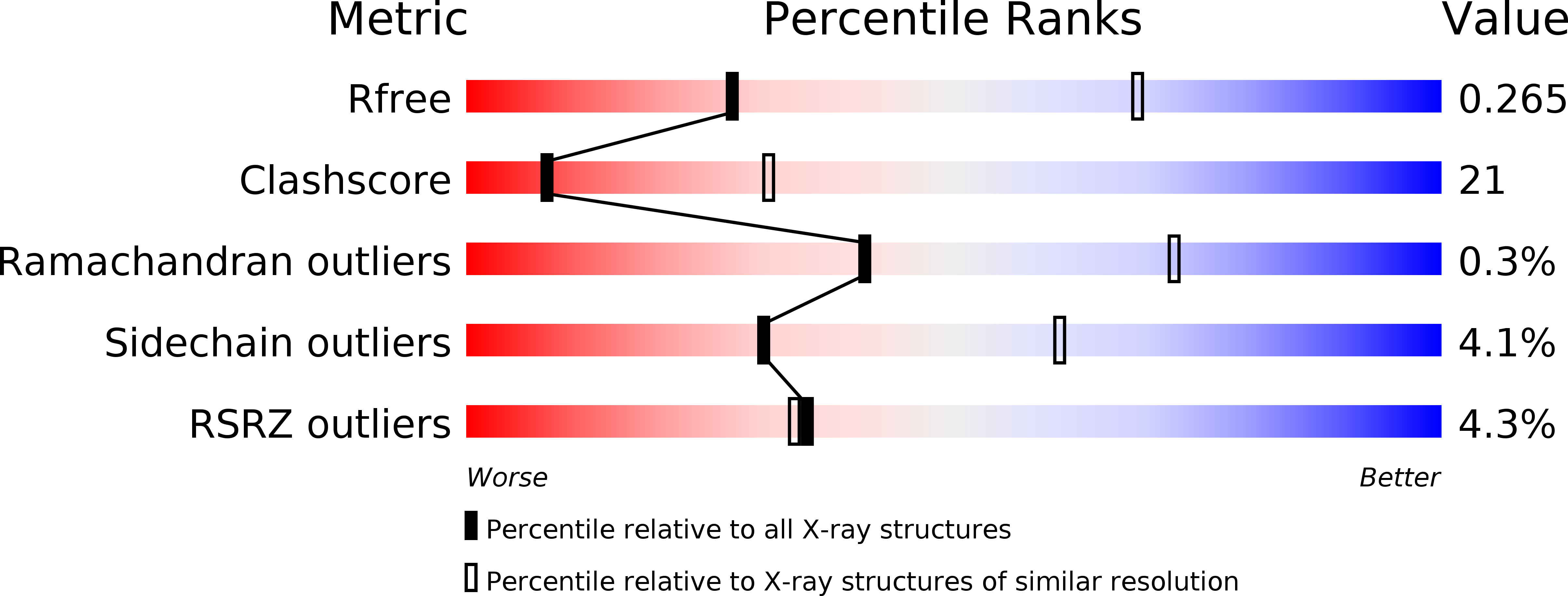
4R26, 4R2G - PubMed Abstract:
The HIV envelope glycoprotein (Env) is densely covered with self-glycans that should help shield it from recognition by the human immune system. Here, we examine how a particularly potent family of broadly neutralizing antibodies (Abs) has evolved common and distinct structural features to counter the glycan shield and interact with both glycan and protein components of HIV Env. The inferred germline antibody already harbors potential binding pockets for a glycan and a short protein segment. Affinity maturation then leads to divergent evolutionary branches that either focus on a single glycan and protein segment (e.g., Ab PGT124) or engage multiple glycans (e.g., Abs PGT121-123). Furthermore, other surrounding glycans are avoided by selecting an appropriate initial antibody shape that prevents steric hindrance. Such molecular recognition lessons are important for engineering proteins that can recognize or accommodate glycans.
Organizational Affiliation:
Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA 92037, USA; International AIDS Vaccine Initiative Neutralizing Antibody Center, The Scripps Research Institute, La Jolla, CA 92037, USA; Scripps Center for HIV/AIDS Vaccine Immunology & Immunogen Discovery, The Scripps Research Institute, La Jolla, CA 92037, USA.