Structure of the Ribosomal Oxygenase OGFOD1 Provides Insights into the Regio- and Stereoselectivity of Prolyl Hydroxylases.
Horita, S., Scotti, J.S., Thinnes, C., Mottaghi-Taromsari, Y.S., Thalhammer, A., Ge, W., Aik, W., Loenarz, C., Schofield, C.J., McDonough, M.A.(2015) Structure 23: 639-652
- PubMed: 25728928
- DOI: https://doi.org/10.1016/j.str.2015.01.014
- Primary Citation of Related Structures:
4NHK, 4NHL, 4NHM, 4NHX, 4NHY - PubMed Abstract:
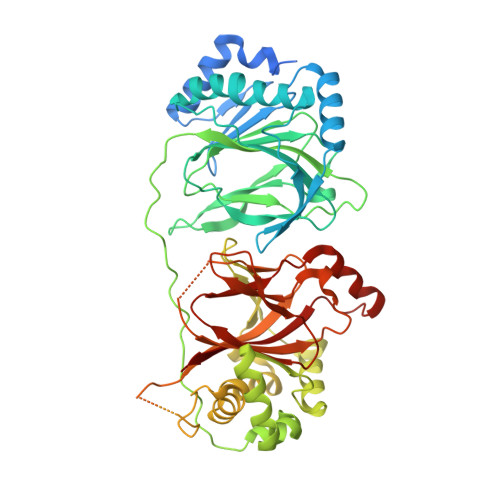
Post-translational ribosomal protein hydroxylation is catalyzed by 2-oxoglutarate (2OG) and ferrous iron dependent oxygenases, and occurs in prokaryotes and eukaryotes. OGFOD1 catalyzes trans-3 prolyl hydroxylation at Pro62 of the small ribosomal subunit protein uS12 (RPS23) and is conserved from yeasts to humans. We describe crystal structures of the human uS12 prolyl 3-hydroxylase (OGFOD1) and its homolog from Saccharomyces cerevisiae (Tpa1p): OGFOD1 in complex with the broad-spectrum 2OG oxygenase inhibitors; N-oxalylglycine (NOG) and pyridine-2,4-dicarboxylate (2,4-PDCA) to 2.1 and 2.6 Å resolution, respectively; and Tpa1p in complex with NOG, 2,4-PDCA, and 1-chloro-4-hydroxyisoquinoline-3-carbonylglycine (a more selective prolyl hydroxylase inhibitor) to 2.8, 1.9, and 1.9 Å resolution, respectively. Comparison of uS12 hydroxylase structures with those of other prolyl hydroxylases, including the human hypoxia-inducible factor (HIF) prolyl hydroxylases (PHDs), reveals differences between the prolyl 3- and prolyl 4-hydroxylase active sites, which can be exploited for developing selective inhibitors of the different subfamilies.
Organizational Affiliation:
Chemistry Research Laboratory, Department of Chemistry, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA, UK; Department of Physiology, Anatomy and Genetics, University of Oxford, Parks Road, Oxford OX1 3PT, UK.