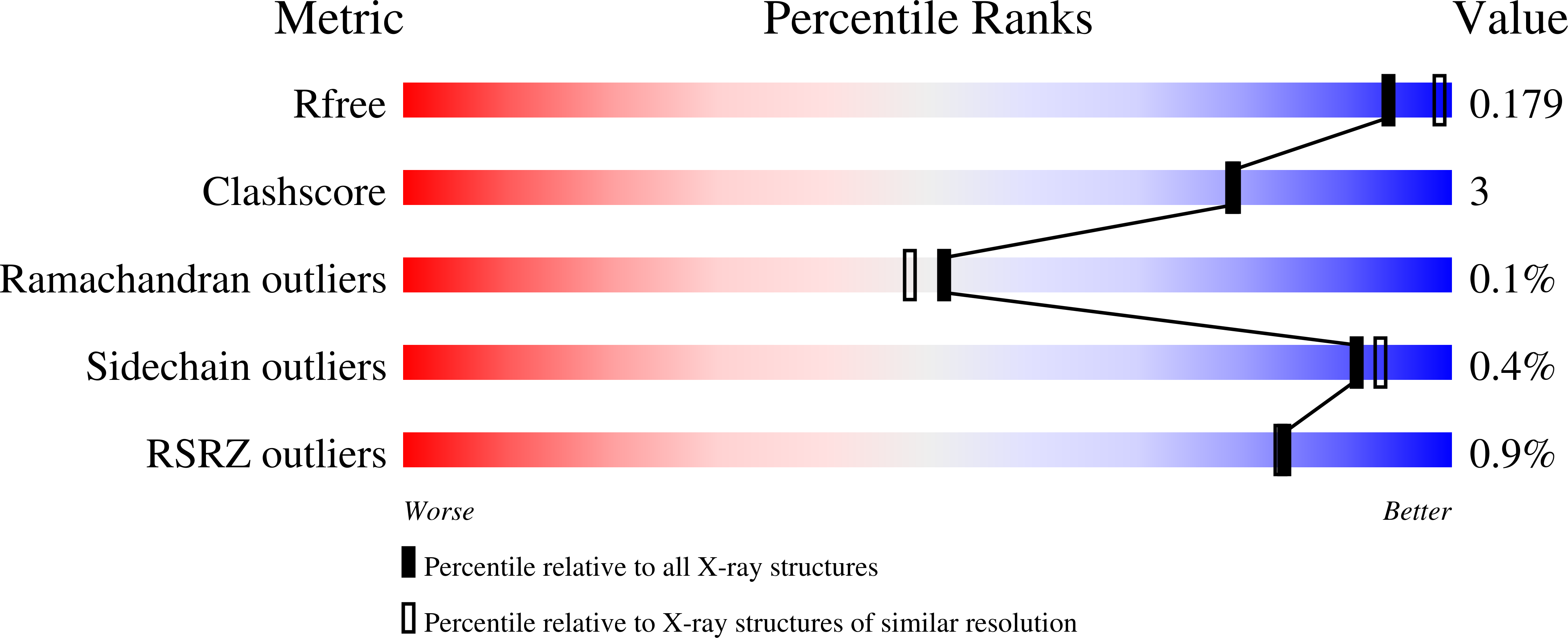
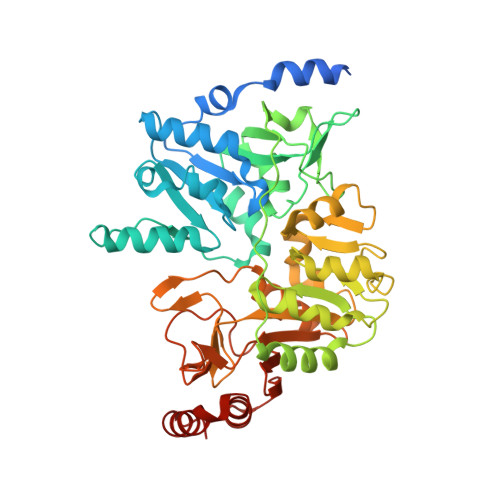
Structural snapshots along the reaction pathway of Yersinia pestis RipA, a putative butyryl-CoA transferase.
Torres, R., Lan, B., Latif, Y., Chim, N., Goulding, C.W.(2014) Acta Crystallogr D Biol Crystallogr 70: 1074-1085
- PubMed: 24699651
- DOI: https://doi.org/10.1107/S1399004714000911
- Primary Citation of Related Structures:
4N8H, 4N8I, 4N8J, 4N8K, 4N8L - PubMed Abstract:
Yersinia pestis, the causative agent of bubonic plague, is able to survive in both extracellular and intracellular environments within the human host, although its intracellular survival within macrophages is poorly understood. A novel Y. pestis three-gene rip (required for intracellular proliferation) operon, and in particular ripA, has been shown to be essential for survival and replication in interferon γ-induced macrophages. RipA was previously characterized as a putative butyryl-CoA transferase proposed to yield butyrate, a known anti-inflammatory shown to lower macrophage-produced NO levels. RipA belongs to the family I CoA transferases, which share structural homology, a conserved catalytic glutamate which forms a covalent CoA-thioester intermediate and a flexible loop adjacent to the active site known as the G(V/I)G loop. Here, functional and structural analyses of several RipA mutants are presented in an effort to dissect the CoA transferase mechanism of RipA. In particular, E61V, M31G and F60M RipA mutants show increased butyryl-CoA transferase activities when compared with wild-type RipA. Furthermore, the X-ray crystal structures of E61V, M31G and F60M RipA mutants, when compared with the wild-type RipA structure, reveal important conformational changes orchestrated by a conserved acyl-group binding-pocket phenylalanine, Phe85, and the G(V/I)G loop. Binary structures of M31G RipA and F60M RipA with two distinct CoA substrate conformations are also presented. Taken together, these data provide CoA transferase reaction snapshots of an open apo RipA, a closed glutamyl-anhydride intermediate and an open CoA-thioester intermediate. Furthermore, biochemical analyses support essential roles for both the catalytic glutamate and the flexible G(V/I)G loop along the reaction pathway, although further research is required to fully understand the function of the acyl-group binding pocket in substrate specificity.
Organizational Affiliation:
Department of Molecular Biology and Biochemistry, UC Irvine, 2212 Natural Sciences I, Irvine, CA 92697, USA.