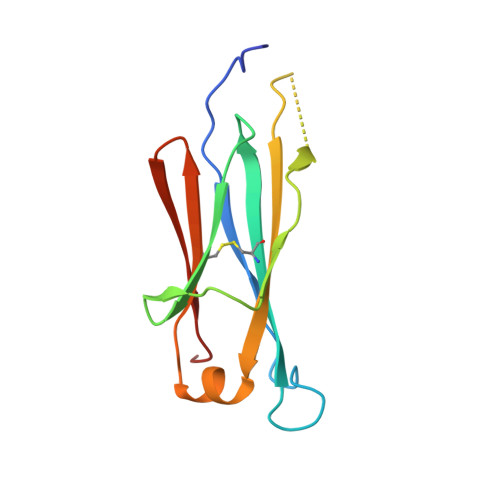
High-resolution structures of the IgM Fc domains reveal principles of its hexamer formation.
Muller, R., Grawert, M.A., Kern, T., Madl, T., Peschek, J., Sattler, M., Groll, M., Buchner, J.(2013) Proc Natl Acad Sci U S A 110: 10183-10188
- PubMed: 23733956
- DOI: https://doi.org/10.1073/pnas.1300547110
- Primary Citation of Related Structures:
4BA8, 4JVU, 4JVW - PubMed Abstract:
IgM is the first antibody produced during the humoral immune response. Despite its fundamental role in the immune system, IgM is structurally only poorly described. In this work we used X-ray crystallography and NMR spectroscopy to determine the atomic structures of the constant IgM Fc domains (Cµ2, Cµ3, and Cµ4) and to address their roles in IgM oligomerization. Although the isolated domains share the typical Ig fold, they differ substantially in dimerization properties and quaternary contacts. Unexpectedly, the Cµ4 domain and its C-terminal tail piece are responsible and sufficient for the specific polymerization of Cµ4 dimers into covalently linked hexamers of dimers. Based on small angle X-ray scattering data, we present a model of the ring-shaped Cµ4 structure, which reveals the principles of IgM oligomerization.
Organizational Affiliation:
Munich Center for Integrated Protein Science at the Department Chemie, Technische Universität München, 85748 Garching, Germany.