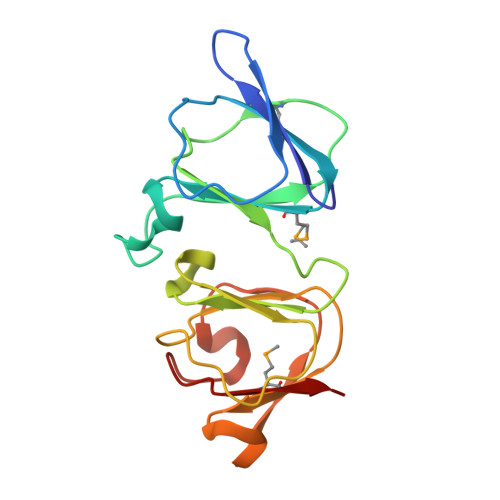
A novel interdomain interface in crystallins: structural characterization of the [beta][gamma]-crystallin from Geodia cydonium at 0.99 A resolution
Vergara, A., Grassi, M., Sica, F., Pizzo, E., D'Alessio, G., Mazzarella, L., Merlino, A.(2013) Acta Crystallogr D Biol Crystallogr 69: 960-967
- PubMed: 23695240
- DOI: https://doi.org/10.1107/S0907444913003569
- Primary Citation of Related Structures:
4IAU - PubMed Abstract:
The βγ-crystallin superfamily includes highly diverse proteins belonging to all of the kingdoms of life. Based on structural topology, these proteins are considered to be evolutionarily related to the long-lived βγ-crystallins that constitute the vertebrate eye lens. This study reports the crystallographic structure at 0.99 Å resolution of the two-domain βγ-crystallin (geodin) from the sponge Geodia cydonium. This is the most ancient member of the βγ-crystallin superfamily in metazoans. The X-ray structure shows that the geodin domains adopt the typical βγ-crystallin fold with a paired Greek-key motif, thus confirming the hypothesis that the crystallin-type scaffold used in the evolution of bacteria and moulds was recruited very early in metazoans. As a significant new structural feature, the sponge protein possesses a unique interdomain interface made up by pairing between the second motif of the first domain and the first motif of the second domain. The atomic resolution also allowed a detailed analysis of the calcium-binding site of the protein.
Organizational Affiliation:
Department of Chemical Sciences, University of Naples 'Federico II', Via Cintia, I-80126 Napoli, Italy.