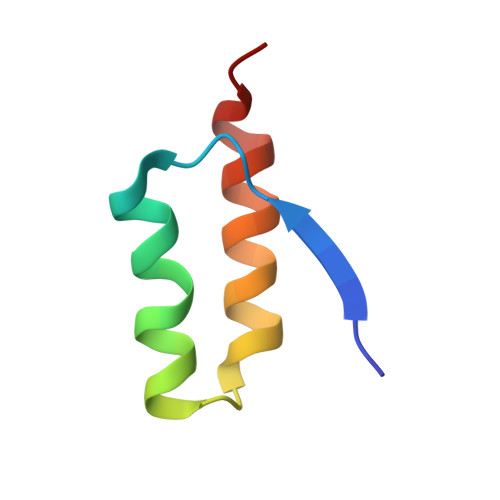
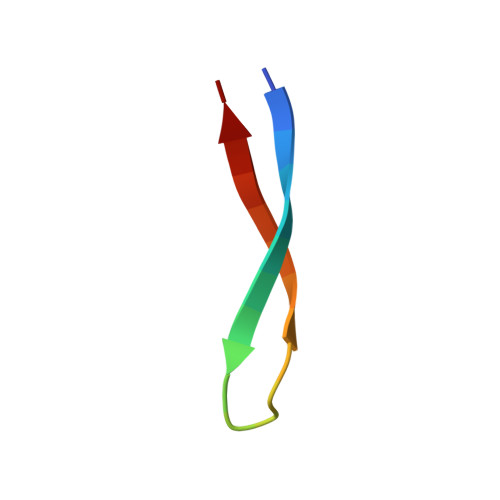
Sequestration of a Beta-Hairpin for Control of Alpha-Synuclein Aggregation.
Mirecka, E.A., Shaykhalishahi, H., Gauhar, A., Akgul, S., Lecher, J., Willbold, D., Stoldt, M., Hoyer, W.(2014) Angew Chem Int Ed Engl 53: 4227
- PubMed: 24623599
- DOI: https://doi.org/10.1002/anie.201309001
- Primary Citation of Related Structures:
4BXL - PubMed Abstract:
The misfolding and aggregation of the protein α-synuclein (α-syn), which results in the formation of amyloid fibrils, is involved in the pathogenesis of Parkinson's disease and other synucleinopathies. The emergence of amyloid toxicity is associated with the formation of partially folded aggregation intermediates. Here, we engineered a class of binding proteins termed β-wrapins (β-wrap proteins) with affinity for α-synuclein (α-syn). The NMR structure of an α-syn:β-wrapin complex reveals a β-hairpin of α-syn comprising the sequence region α-syn(37-54). The β-wrapin inhibits α-syn aggregation and toxicity at substoichiometric concentrations, demonstrating that it interferes with the nucleation of aggregation.
Organizational Affiliation:
Institut für Physikalische Biologie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf (Germany).